ORTHOFLASH®
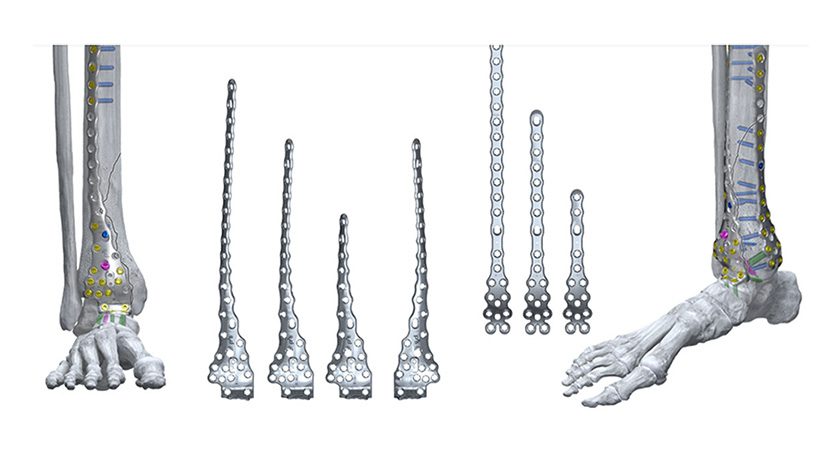
The company announced the full commercial launch of the Gorilla Pilon Fusion Plating System and the Phantom TTC Trauma Nail.
ALAYA is a CE-certified surgical robotic assistant for spine with patented Kinematic Navigation Technology.
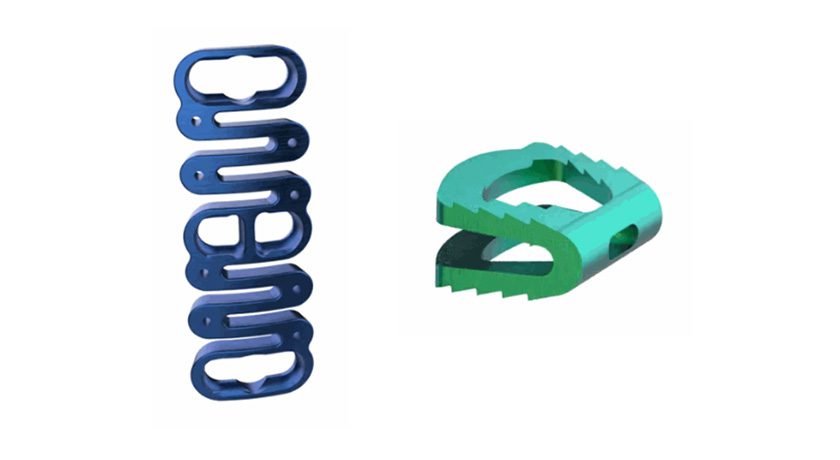
Study participants received the company’s next generation CEM-Plate and CEM-Cage used in anterior cervical discectomy and fusion procedures.
October 6, 2025
Patrick McGuire
October 2, 2025
,
Julie A. Vetalice
September 26, 2025
,
Julie A. Vetalice
The company announced the full commercial launch of the Gorilla Pilon Fusion Plating System and the Phantom TTC Trauma Nail.
October 8, 2025
,
ALAYA is a CE-certified surgical robotic assistant for spine with patented Kinematic Navigation Technology.
October 8, 2025
,
Study participants received the company’s next generation CEM-Plate and CEM-Cage used in anterior cervical discectomy and fusion procedures.
October 7, 2025
,
TigerShark Lateral uses the proprietary BioBond porous trabecular structure to create the new lateral interbody system.
October 6, 2025
,
Introduction of the pre-op planning and modeling system completes the CORIOGRAPH Services portfolio, which includes solutions for knee and hip arthroplasty.
October 2, 2025
,
Bioretec’s RemeOs screw is the first and only osteopromotive absorbable metal implant for orthopedic use in the U.S.
October 1, 2025
,
The iTaperloc Complete and iG7 Hip System with Iodine Technology inhibits bacterial adhesion and prevents biofilm formation on the implant surface to address PJI.
September 26, 2025
,
With a tiny, high-quality camera on the tip of a needle-like device, the scope requires only a small incision yielding minimal tissue disruption and a smaller scar.
September 23, 2025
,
Shoulder Innovations’ InSet 70 addresses osteoarthritis and avascular necrosis, rheumatoid and traumatic arthritis, and correction of functional deformity.
September 22, 2025
,
By combining novel low-dose fluoroscopic hardware with an AI-enabled imaging solution, ODI addresses the limitations of 2D x-ray and CT systems.
September 19, 2025
,
Spine Mixed Reality Navigation combines the precision of the company’s signature optical navigation system with advanced mixed reality.
September 18, 2025
,
DEXA-L is a first-of-its-kind standalone device for use in anterior lumbar interbody fusion procedures.
September 18, 2025
,
The device is designed to work in tandem with Ruthless Spine’s De Novo cleared RJB intraoperative angle measurement instrument.
September 18, 2025
,
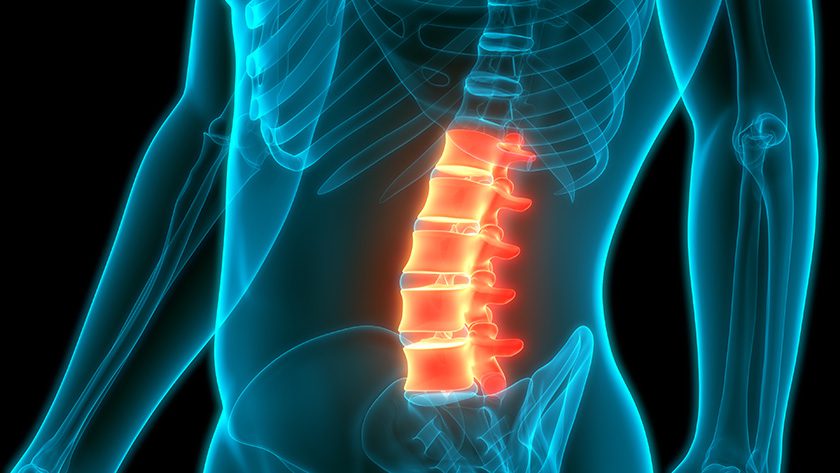
The VCFix Spinal System is a vertebral augmentation system designed to treat a broad spectrum of vertebral compression fractures.
September 17, 2025
,
Medartis’ TOUCH implant addresses a clinical need in hand surgery, as the CMC 1 joint is among the most commonly affected by osteoarthritis.