
 Copy to clipboard
Copy to clipboard 
Companion Spine raised $55 million. This Series A financing was led by Viscogliosi Brothers. Funds will primarily support the development and commercialization of minimally invasive diagnostic and therapeutic solutions to treat degenerative disc disease and lumbar spinal stenosis.
The company’s products, stemming in part from an acquisition from Medtronic in 2020 of regulatory and clinical property, includes a portfolio of six patent families covering more than 140 patents, as well as an inventory of instrument and implant sets. These technologies may offer an alternative to options such as spine fusion.
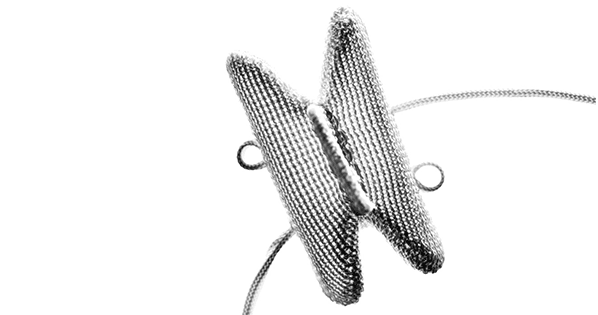
Two of Companion Spine’s products are expected to be commercialized in the U.S. in 2023. The DIAM implant for the treatment of degenerative disc disease is already CE Marked. It received the Breakthrough Device designation from FDA in 2021. The second product is the APERIUS intervertebral implant for the percutaneous treatment of lumbar stenosis.
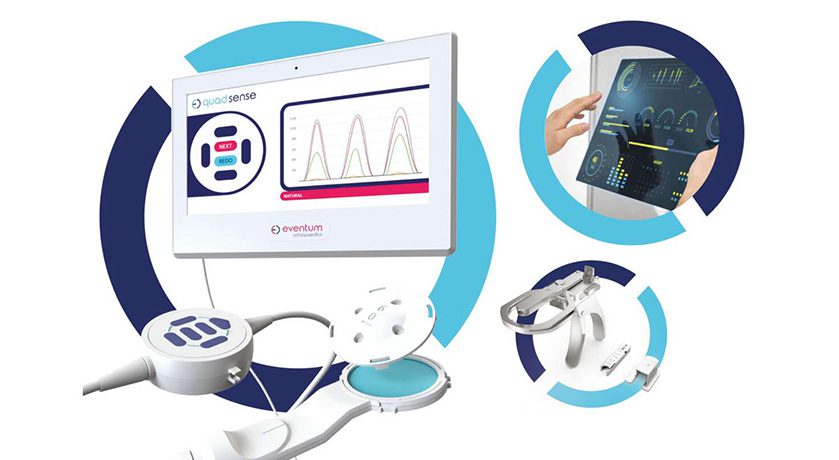
Erick Cloix, CEO and co-founder of Companion Spine, said, “Companion Spine will be able to focus entirely on its first group of medical, diagnostic, and implantable devices, specifically DIAM and APERIUS, as well as DISC ANALYZER, a new technology for the precise diagnosis and monitoring of back pain. United States commercialization of these innovations is expected during 2023 and 2024.”
Companion Spine is in discussions with several companies to establish potential partnerships and acquisitions of complementary products and technologies.
Source: Companion Spine
Companion Spine raised $55 million. This Series A financing was led by Viscogliosi Brothers. Funds will primarily support the development and commercialization of minimally invasive diagnostic and therapeutic solutions to treat degenerative disc disease and lumbar spinal stenosis.
The company's products, stemming in part from an acquisition...
Companion Spine raised $55 million. This Series A financing was led by Viscogliosi Brothers. Funds will primarily support the development and commercialization of minimally invasive diagnostic and therapeutic solutions to treat degenerative disc disease and lumbar spinal stenosis.
The company’s products, stemming in part from an acquisition from Medtronic in 2020 of regulatory and clinical property, includes a portfolio of six patent families covering more than 140 patents, as well as an inventory of instrument and implant sets. These technologies may offer an alternative to options such as spine fusion.
Two of Companion Spine’s products are expected to be commercialized in the U.S. in 2023. The DIAM implant for the treatment of degenerative disc disease is already CE Marked. It received the Breakthrough Device designation from FDA in 2021. The second product is the APERIUS intervertebral implant for the percutaneous treatment of lumbar stenosis.
Erick Cloix, CEO and co-founder of Companion Spine, said, “Companion Spine will be able to focus entirely on its first group of medical, diagnostic, and implantable devices, specifically DIAM and APERIUS, as well as DISC ANALYZER, a new technology for the precise diagnosis and monitoring of back pain. United States commercialization of these innovations is expected during 2023 and 2024.”
Companion Spine is in discussions with several companies to establish potential partnerships and acquisitions of complementary products and technologies.
Source: Companion Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.