
 Copy to clipboard
Copy to clipboard 
ReVivo Medical announced completion of the first two surgical procedures using the company’s new design of anterior cervical plates and interbody cages. “The procedures are part of our FDA sanctioned IDE clinical trial, a requirement for attaining regulatory clearance prior to offering a commercial product,” said Gary Mittleman, President and CEO. “Although final clearance may be as far off as 2024, we are now well on our way.”
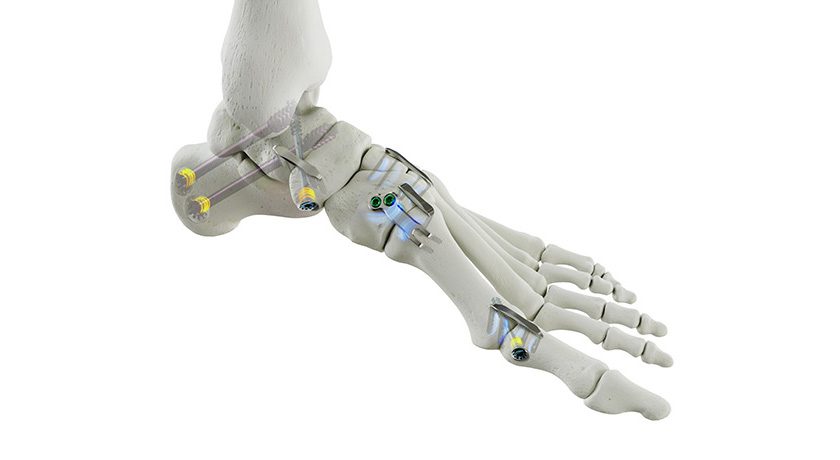
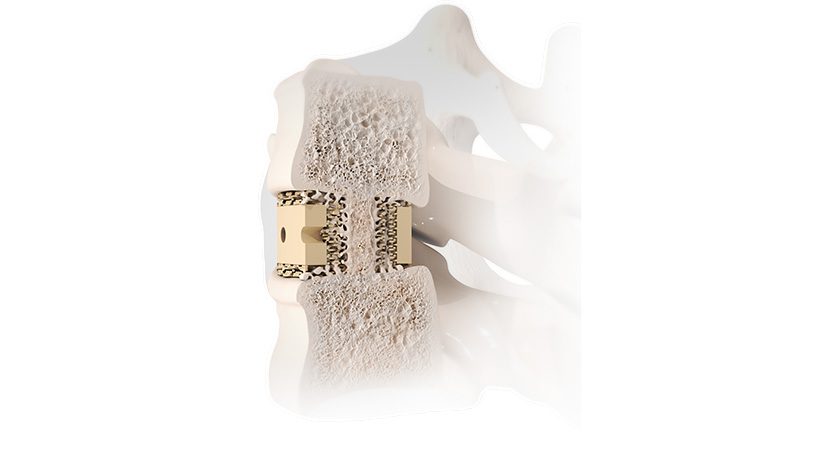
Study participants will receive ReVivo Medical’s next-generation cervical plate and interbody cages used in anterior cervical discectomy and fusion procedures.
“Through the clinical trial, we hope to demonstrate that our cervical plate and cage implants improve bone formation and achieve a superior rate and quality of fusion as compared to the commonly used devices of today,” said Eric Ledet, Ph.D., Chief Science Officer. “Additionally, we will determine if the designs of our implants make them easier for the surgeon to use.”
None of ReVivo Medical’s devices are currently cleared for use in the United States.
Source: ReVivo Medical
ReVivo Medical announced completion of the first two surgical procedures using the company’s new design of anterior cervical plates and interbody cages. “The procedures are part of our FDA sanctioned IDE clinical trial, a requirement for attaining regulatory clearance prior to offering a commercial product,” said Gary Mittleman, President and...
ReVivo Medical announced completion of the first two surgical procedures using the company’s new design of anterior cervical plates and interbody cages. “The procedures are part of our FDA sanctioned IDE clinical trial, a requirement for attaining regulatory clearance prior to offering a commercial product,” said Gary Mittleman, President and CEO. “Although final clearance may be as far off as 2024, we are now well on our way.”
Study participants will receive ReVivo Medical’s next-generation cervical plate and interbody cages used in anterior cervical discectomy and fusion procedures.
“Through the clinical trial, we hope to demonstrate that our cervical plate and cage implants improve bone formation and achieve a superior rate and quality of fusion as compared to the commonly used devices of today,” said Eric Ledet, Ph.D., Chief Science Officer. “Additionally, we will determine if the designs of our implants make them easier for the surgeon to use.”
None of ReVivo Medical’s devices are currently cleared for use in the United States.
Source: ReVivo Medical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.