
 Copy to clipboard
Copy to clipboard 
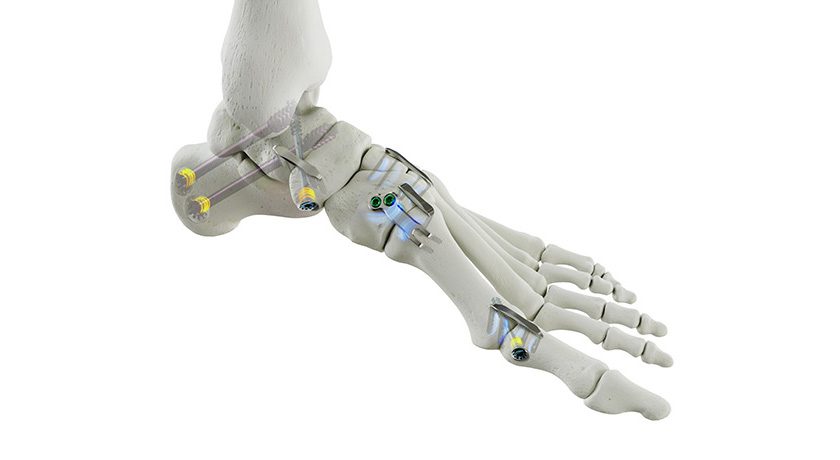
OSSIO announced U.S. launch and first commercial use of OSSIOfiber® Suture Anchors for use in foot/ankle, shoulder, knee, hand/wrist and elbow surgery.
OSSIOfiber Suture Anchors were FDA-cleared in March 2022 for use in fixation of suture/soft tissue to bone in the shoulder, foot/ankle, knee, hand/wrist and elbow in a variety of orthopedic procedures. These implants were designed using proprietary OSSIOfiber Intelligent Bone Regeneration Technology that leaves nothing permanent behind.
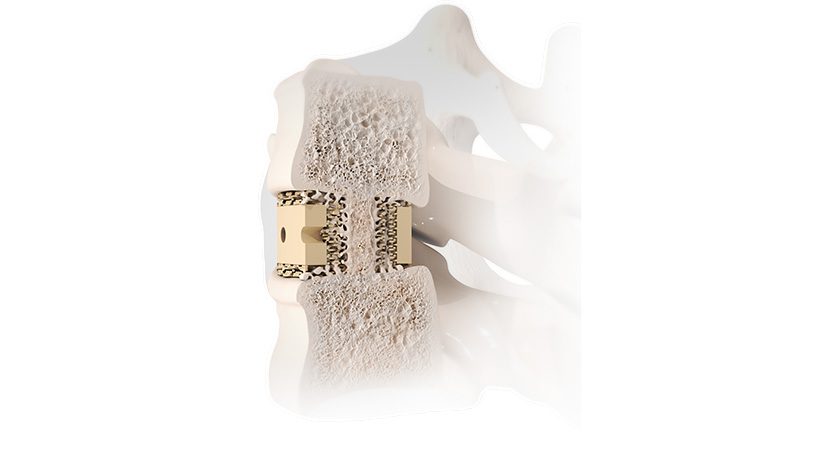
Designed for rapid bone in-growth, regeneration and replacement, OSSIOfiber Intelligent Bone Regeneration Technology is a first-of-its-kind implant material stronger than cortical bone. OSSIOfiber is engineered to provide the strength required for functional fixation and allows for full integration into the native anatomy without adverse biological response. OSSIOfiber implants utilize existing reimbursement and surgical techniques.
Additionally, the anchor’s proprietary DURAlink™ Coupling Technology acts to increase stability and prevent suture slippage by connecting the eyelet to the anchor, creating a single stable unit. OSSIO will initially launch the 4.75mm system into the foot and ankle market, followed by the company’s first entry into the sports/shoulder markets later this year.
OSSIO CEO Brian Verrier said, “Sports and extremity surgeons have been asking for non-permanent suture anchors that deliver improved strength and pull-out resistance, while providing safe, predictable bio-integration. OSSIOfiber Suture Anchors have shown strength that is unrivaled and safety that is unmatched in the market, as demonstrated by our bench testing and 30-month in-life studies compared to currently marketed bio-composite anchor controls.”
“Additionally,” Verrier said, “our products offer enhanced ease of use due to the incredible insertion strength of our continuous mineral fiber-based platform. We are excited to offer our customers a suture anchor that provides confidence during the healing process while avoiding many of the late issues sometimes seen in traditional suture anchors.”
Source: OSSIO
OSSIO announced U.S. launch and first commercial use of OSSIOfiber® Suture Anchors for use in foot/ankle, shoulder, knee, hand/wrist and elbow surgery.
OSSIOfiber Suture Anchors were FDA-cleared in March 2022 for use in fixation of suture/soft tissue to bone in the shoulder, foot/ankle, knee, hand/wrist and elbow in a variety of orthopedic...
OSSIO announced U.S. launch and first commercial use of OSSIOfiber® Suture Anchors for use in foot/ankle, shoulder, knee, hand/wrist and elbow surgery.
OSSIOfiber Suture Anchors were FDA-cleared in March 2022 for use in fixation of suture/soft tissue to bone in the shoulder, foot/ankle, knee, hand/wrist and elbow in a variety of orthopedic procedures. These implants were designed using proprietary OSSIOfiber Intelligent Bone Regeneration Technology that leaves nothing permanent behind.
Designed for rapid bone in-growth, regeneration and replacement, OSSIOfiber Intelligent Bone Regeneration Technology is a first-of-its-kind implant material stronger than cortical bone. OSSIOfiber is engineered to provide the strength required for functional fixation and allows for full integration into the native anatomy without adverse biological response. OSSIOfiber implants utilize existing reimbursement and surgical techniques.
Additionally, the anchor’s proprietary DURAlink™ Coupling Technology acts to increase stability and prevent suture slippage by connecting the eyelet to the anchor, creating a single stable unit. OSSIO will initially launch the 4.75mm system into the foot and ankle market, followed by the company’s first entry into the sports/shoulder markets later this year.
OSSIO CEO Brian Verrier said, “Sports and extremity surgeons have been asking for non-permanent suture anchors that deliver improved strength and pull-out resistance, while providing safe, predictable bio-integration. OSSIOfiber Suture Anchors have shown strength that is unrivaled and safety that is unmatched in the market, as demonstrated by our bench testing and 30-month in-life studies compared to currently marketed bio-composite anchor controls.”
“Additionally,” Verrier said, “our products offer enhanced ease of use due to the incredible insertion strength of our continuous mineral fiber-based platform. We are excited to offer our customers a suture anchor that provides confidence during the healing process while avoiding many of the late issues sometimes seen in traditional suture anchors.”
Source: OSSIO

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.