
 Copy to clipboard
Copy to clipboard 
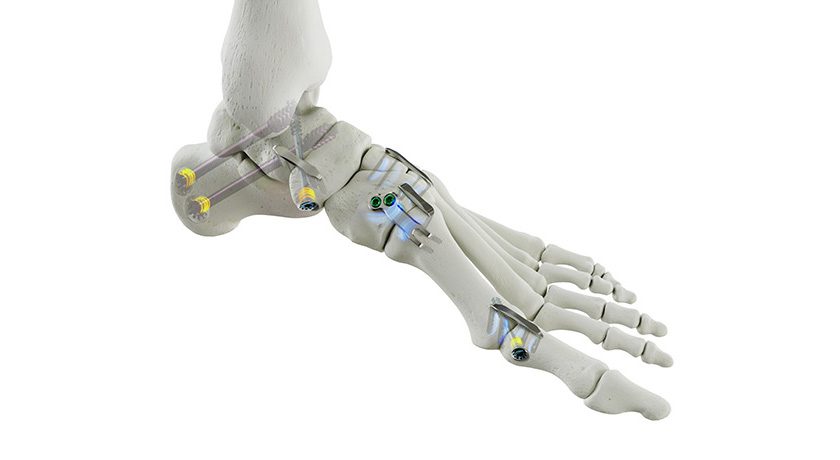
VYRSA Technologies launched the FDA-cleared VYRSA V1™ SI Fusion System.
According to data from the biomechanical testing reports for SI joint fixation, no significant differences were found between the Test, VYRSA V1™, and control implant groups with respect to any of the outcome measures investigated. The report concluded that the lack of a statistically significant difference between the two techniques evaluated in this study is consistent with previous studies comparing the lateral and posterior approaches to SIJ fusion.
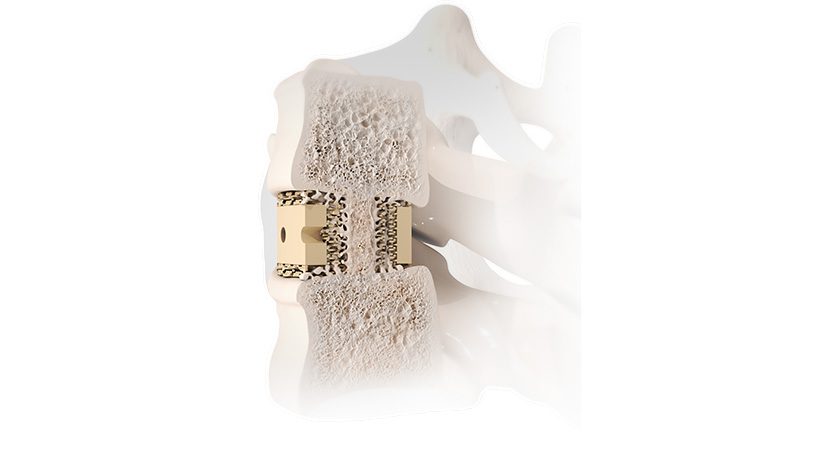
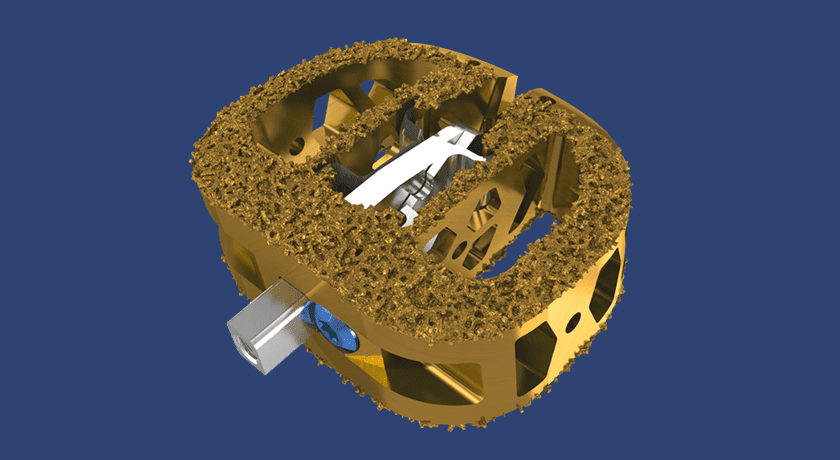
VYRSA V1 is a fusion device comprising a 3D-printed titanium body with a roughened surface designed to encourage bone growth onto the surfaces of the device. The V1 implant surfaces are engineered with pores that average 500 microns in diameter to support the incorporation of the implant within the sacroiliac joint. VYRSA V1 has multiple openings to allow a large volume of autogenous bone graft to be easily packed into the implant to facilitate fusion. The device features two sharpened anchor plates housed within the 3D-printed body until they are deployed into the ilium and sacrum to provide fixation.
Terry Harvey, President of VYRSA Technologies, said, “The entry of the VYRSA V1 implant into the U.S. market represents a huge step for VYRSA in continuing to provide innovative, physician-friendly solutions for the treatment of sacroiliac joint dysfunction. The FDA 510(k) clearance is a very exciting step forward for patients who suffer from pain due to sacroiliac joint disruptions and degenerative sacroiliitis.”
Source: VYRSA Technologies
VYRSA Technologies launched the FDA-cleared VYRSA V1™ SI Fusion System.
According to data from the biomechanical testing reports for SI joint fixation, no significant differences were found between the Test, VYRSA V1™, and control implant groups with respect to any of the outcome measures investigated. The report concluded that the lack of a...
VYRSA Technologies launched the FDA-cleared VYRSA V1™ SI Fusion System.
According to data from the biomechanical testing reports for SI joint fixation, no significant differences were found between the Test, VYRSA V1™, and control implant groups with respect to any of the outcome measures investigated. The report concluded that the lack of a statistically significant difference between the two techniques evaluated in this study is consistent with previous studies comparing the lateral and posterior approaches to SIJ fusion.
VYRSA V1 is a fusion device comprising a 3D-printed titanium body with a roughened surface designed to encourage bone growth onto the surfaces of the device. The V1 implant surfaces are engineered with pores that average 500 microns in diameter to support the incorporation of the implant within the sacroiliac joint. VYRSA V1 has multiple openings to allow a large volume of autogenous bone graft to be easily packed into the implant to facilitate fusion. The device features two sharpened anchor plates housed within the 3D-printed body until they are deployed into the ilium and sacrum to provide fixation.
Terry Harvey, President of VYRSA Technologies, said, “The entry of the VYRSA V1 implant into the U.S. market represents a huge step for VYRSA in continuing to provide innovative, physician-friendly solutions for the treatment of sacroiliac joint dysfunction. The FDA 510(k) clearance is a very exciting step forward for patients who suffer from pain due to sacroiliac joint disruptions and degenerative sacroiliitis.”
Source: VYRSA Technologies

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.