
 Copy to clipboard
Copy to clipboard 
Orthofix Medical announced results from a prospective, multicenter clinical study evaluating the safety and efficacy of the Trinity Elite™ cellular bone allograft (CBA) in lumbar spinal fusion. Patients treated with the Trinity Elite CBA demonstrated fusion rates of 98.6%, measured by bridging bone at 12 months follow-up.
In this open-label clinical study, Trinity Elite allograft was evaluated in subjects undergoing posterolateral fusion (1-4 levels) or interbody fusion (1-2 levels) with CBA. Subject risk factors included smoking, diabetes, obesity and osteoporosis. A total of 274 subjects were enrolled in the study, with available data from 201 subjects who completed 12-month follow-up. Radiographic fusion status was assessed by an independent review of dynamic radiographs and CT scans.
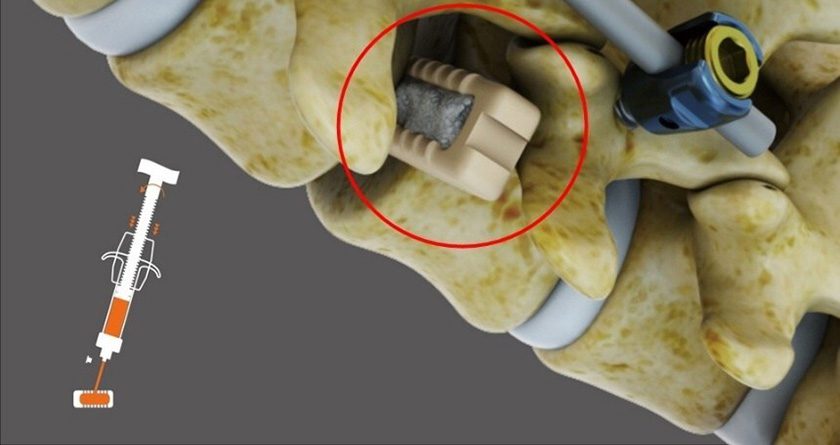
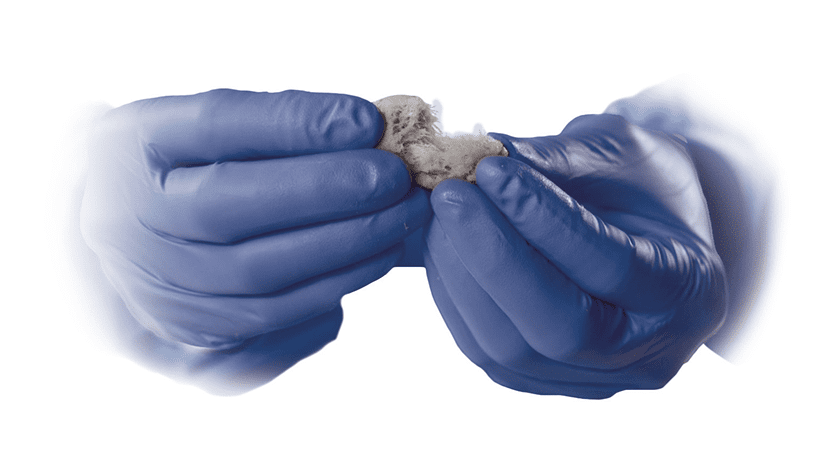
Trinity Elite is a cryopreserved CBA from allograft donor bone that facilitates bone formation by providing an osteoconductive scaffold, inherent osteoinductive growth factors and osteogenic cells. Trinity Elite eliminates the need for harvesting autograft from patients. Processed by MTF Biologics, Trinity Elite moldable bone graft material enables physicians to easily control the placement of tissue during procedures.
“We are pleased to continue to invest in clinical research and provide physicians the information they need to make the best decisions for their patients,” said Orthofix President of Global Spine Kevin Kenny. “The results of this publication support Trinity Elite as a safe and efficacious alternative to autograft for patients undergoing lumbar fusion procedures and demonstrate the compelling benefits of this cellular bone allograft.”
Source: Orthofix Medical
Orthofix Medical announced results from a prospective, multicenter clinical study evaluating the safety and efficacy of the Trinity Elite™ cellular bone allograft (CBA) in lumbar spinal fusion. Patients treated with the Trinity Elite CBA demonstrated fusion rates of 98.6%, measured by bridging bone at 12 months follow-up.
In this open-label...
Orthofix Medical announced results from a prospective, multicenter clinical study evaluating the safety and efficacy of the Trinity Elite™ cellular bone allograft (CBA) in lumbar spinal fusion. Patients treated with the Trinity Elite CBA demonstrated fusion rates of 98.6%, measured by bridging bone at 12 months follow-up.
In this open-label clinical study, Trinity Elite allograft was evaluated in subjects undergoing posterolateral fusion (1-4 levels) or interbody fusion (1-2 levels) with CBA. Subject risk factors included smoking, diabetes, obesity and osteoporosis. A total of 274 subjects were enrolled in the study, with available data from 201 subjects who completed 12-month follow-up. Radiographic fusion status was assessed by an independent review of dynamic radiographs and CT scans.
Trinity Elite is a cryopreserved CBA from allograft donor bone that facilitates bone formation by providing an osteoconductive scaffold, inherent osteoinductive growth factors and osteogenic cells. Trinity Elite eliminates the need for harvesting autograft from patients. Processed by MTF Biologics, Trinity Elite moldable bone graft material enables physicians to easily control the placement of tissue during procedures.
“We are pleased to continue to invest in clinical research and provide physicians the information they need to make the best decisions for their patients,” said Orthofix President of Global Spine Kevin Kenny. “The results of this publication support Trinity Elite as a safe and efficacious alternative to autograft for patients undergoing lumbar fusion procedures and demonstrate the compelling benefits of this cellular bone allograft.”
Source: Orthofix Medical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.