
 Copy to clipboard
Copy to clipboard 
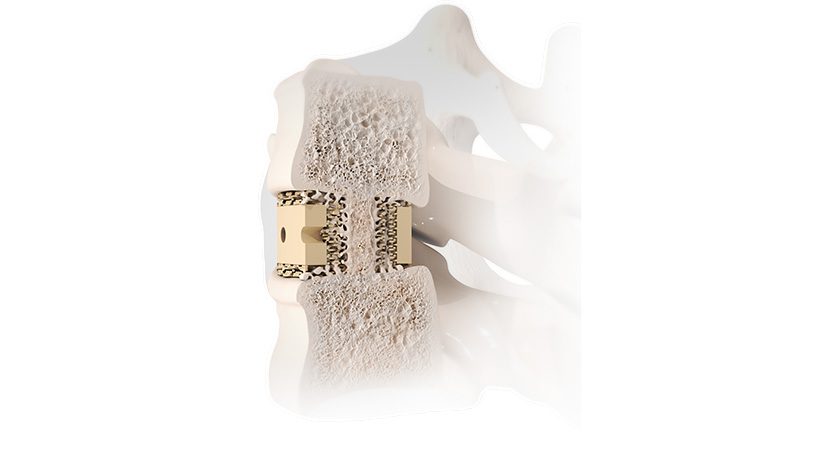
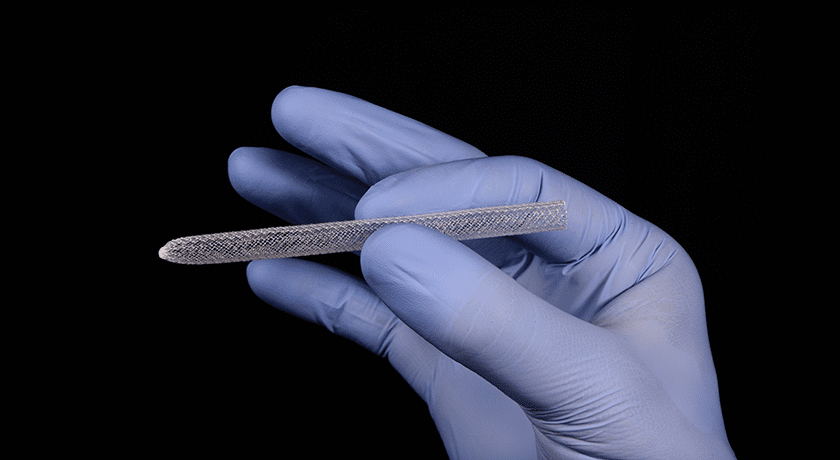
Woven Orthopedic Technologies was granted FDA 510(k) clearance to market its Ogmend Implant Enhancement System for use in spine surgery. Ogmend is an implantable sleeve designed to aid screw fixation in challenging scenarios.
Ogmend will be made available in the U.S. through a staged, regional release.
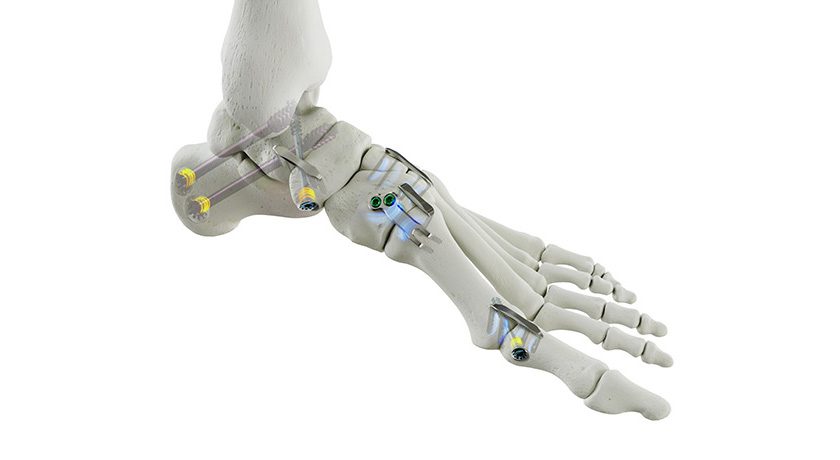
Orthopedic surgeons place more than one billion screws each year to hold broken bones in position during the healing process. However, surgeons routinely face scenarios that make it difficult to achieve secure fixation between screws and bone. In those situations, the Ogmend Sleeve offers an off-the-shelf solution to hold screws in place. It can be deployed in under two minutes and is used with a wide range of screw systems from a variety of suppliers.
Ogmend, which is also cleared under the CE Mark for use in spine surgery, recently eclipsed the 2,500-unit milestone in Europe. In 2020, Ogmend received FDA de novo clearance for use in the U.S. in long bone trauma surgery.
“We are thrilled to deliver surgeons a simple, reliable solution to help overcome one of the most prevalent yet least discussed challenges in orthopedic and spine surgery: the ability to more confidently use surgical screws when operating in compromised fixation scenarios,” explained Woven Orthopedics’ Co-founder and President, Brandon Bendes.
Sources: Woven Orthopedic Technologies; FDA.gov
Woven Orthopedic Technologies was granted FDA 510(k) clearance to market its Ogmend Implant Enhancement System for use in spine surgery. Ogmend is an implantable sleeve designed to aid screw fixation in challenging scenarios.
Ogmend will be made available in the U.S. through a staged, regional release.
Orthopedic surgeons place more than...
Woven Orthopedic Technologies was granted FDA 510(k) clearance to market its Ogmend Implant Enhancement System for use in spine surgery. Ogmend is an implantable sleeve designed to aid screw fixation in challenging scenarios.
Ogmend will be made available in the U.S. through a staged, regional release.
Orthopedic surgeons place more than one billion screws each year to hold broken bones in position during the healing process. However, surgeons routinely face scenarios that make it difficult to achieve secure fixation between screws and bone. In those situations, the Ogmend Sleeve offers an off-the-shelf solution to hold screws in place. It can be deployed in under two minutes and is used with a wide range of screw systems from a variety of suppliers.
Ogmend, which is also cleared under the CE Mark for use in spine surgery, recently eclipsed the 2,500-unit milestone in Europe. In 2020, Ogmend received FDA de novo clearance for use in the U.S. in long bone trauma surgery.
“We are thrilled to deliver surgeons a simple, reliable solution to help overcome one of the most prevalent yet least discussed challenges in orthopedic and spine surgery: the ability to more confidently use surgical screws when operating in compromised fixation scenarios,” explained Woven Orthopedics’ Co-founder and President, Brandon Bendes.
Sources: Woven Orthopedic Technologies; FDA.gov

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.