
 Copy to clipboard
Copy to clipboard 
Relievant Medsystems announced the publication of three-year pooled results from two prospective clinical trials that further validate the safety, effectiveness and long-term durability of the Intracept Procedure for patients with vertebrogenic pain. Results are consistent with previously published long-term results that measured Intracept Procedure outcomes at five years.
The study includes 95 patients across 22 study sites who were successfully treated with the Intracept Procedure and completed three-year study visits. These patients achieved statistically significant, clinically meaningful and durable improvements in both pain and function.
At three years post-Intracept Procedure, statistically significant improvements of 31.2 points and 4.3 points were observed compared to baseline, for mean Oswestry Disability Index and mean numeric pain score, respectively. The study also found that 74% fewer patients were actively using opioid medications for low back pain, and 84% fewer patients required spinal injections for the same pain source and treatment level at three years following treatment.
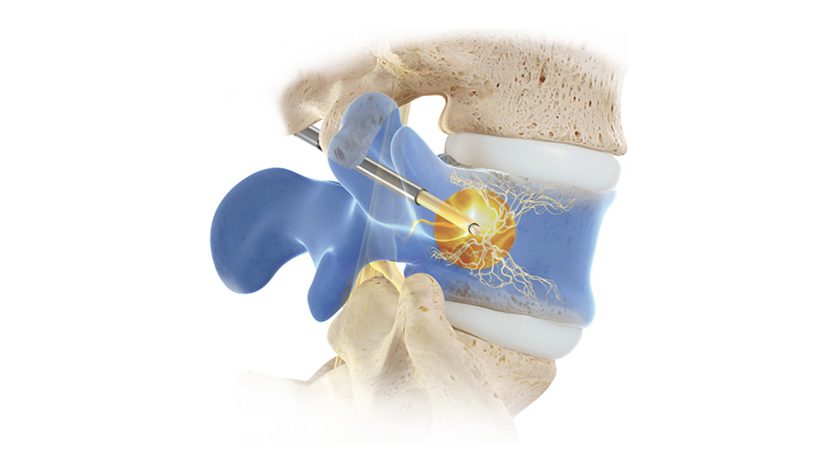
Relievant Medsystems’ minimally invasive Intracept Procedure is the only FDA-cleared treatment for chronic vertebrogenic low back pain. The same-day, outpatient procedure uses targeted radiofrequency energy to stop the basivertebral nerve from transmitting pain signals to the brain and takes approximately one hour to perform.
Other key findings at three years post-procedure include:
- 26.3% of patients reported being 100% pain-free
- 71% of patients indicated they had returned to the level of activity they enjoyed prior to experiencing chronic low back pain
- 86% of patients indicated they would have the procedure again for the same condition
- No serious device or device-procedure related adverse events were reported, highlighting the safety of this procedure
“We are encouraged to see positive outcomes consistent with results from multiple clinical trials, including two Level I randomized controlled trials, that demonstrate the long-term durability of the Intracept Procedure,” said Tyler Binney, President and CEO of Relievant Medsystems. “These outcomes represent improved quality of life for patients and we are committed to continuing to provide vertebrogenic pain relief to the millions of individuals with this often debilitating condition.”
Source: Relievant Medsystems
Relievant Medsystems announced the publication of three-year pooled results from two prospective clinical trials that further validate the safety, effectiveness and long-term durability of the Intracept Procedure for patients with vertebrogenic pain. Results are consistent with previously published long-term results that measured Intracept...
Relievant Medsystems announced the publication of three-year pooled results from two prospective clinical trials that further validate the safety, effectiveness and long-term durability of the Intracept Procedure for patients with vertebrogenic pain. Results are consistent with previously published long-term results that measured Intracept Procedure outcomes at five years.
The study includes 95 patients across 22 study sites who were successfully treated with the Intracept Procedure and completed three-year study visits. These patients achieved statistically significant, clinically meaningful and durable improvements in both pain and function.
At three years post-Intracept Procedure, statistically significant improvements of 31.2 points and 4.3 points were observed compared to baseline, for mean Oswestry Disability Index and mean numeric pain score, respectively. The study also found that 74% fewer patients were actively using opioid medications for low back pain, and 84% fewer patients required spinal injections for the same pain source and treatment level at three years following treatment.
Relievant Medsystems’ minimally invasive Intracept Procedure is the only FDA-cleared treatment for chronic vertebrogenic low back pain. The same-day, outpatient procedure uses targeted radiofrequency energy to stop the basivertebral nerve from transmitting pain signals to the brain and takes approximately one hour to perform.
Other key findings at three years post-procedure include:
- 26.3% of patients reported being 100% pain-free
- 71% of patients indicated they had returned to the level of activity they enjoyed prior to experiencing chronic low back pain
- 86% of patients indicated they would have the procedure again for the same condition
- No serious device or device-procedure related adverse events were reported, highlighting the safety of this procedure
“We are encouraged to see positive outcomes consistent with results from multiple clinical trials, including two Level I randomized controlled trials, that demonstrate the long-term durability of the Intracept Procedure,” said Tyler Binney, President and CEO of Relievant Medsystems. “These outcomes represent improved quality of life for patients and we are committed to continuing to provide vertebrogenic pain relief to the millions of individuals with this often debilitating condition.”
Source: Relievant Medsystems

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.