Copy to clipboard
Copy to clipboard 
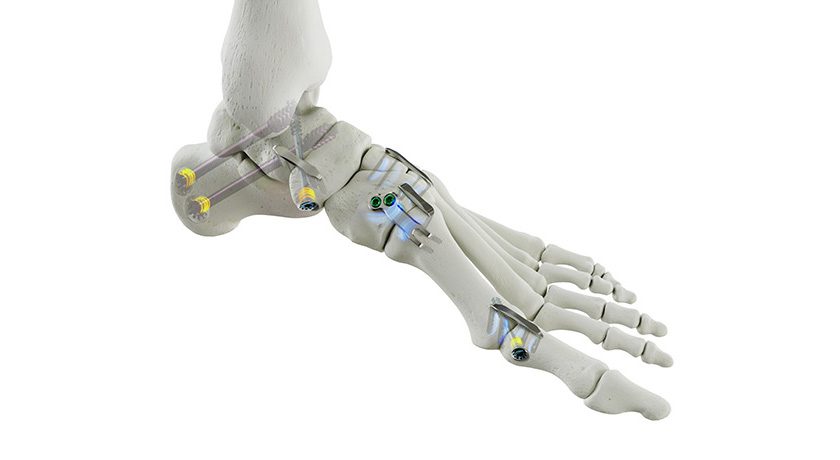
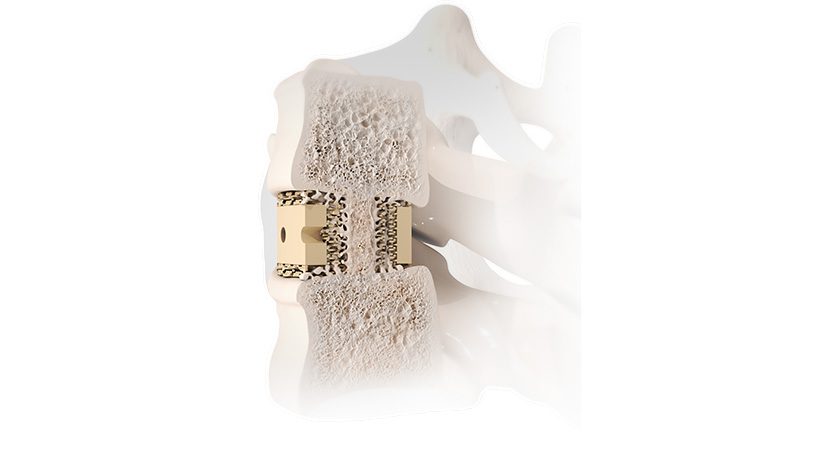
SI-BONE announced the first cases completed with the smaller, 9.5 mm diameter iFuse Bedrock Granite Implant (Granite 9.5). When placed across the SI joint, the Granite implant provides sacroiliac fusion and spinopelvic fixation as a foundational element for multi-segment spinal fusion.
This milestone for Granite 9.5 comes after FDA 510(k) marketing clearance in January 2024. The iFuse Bedrock Granite Implant System was awarded a Breakthrough Device Designation by FDA and a New Technology Add-on Payment by the Centers for Medicare and Medicaid Services. Granite 9.5 may be more suitable for patients with smaller anatomy and may allow easier placement of stacked implants in the sacroalar-iliac trajectory. Moreover, the recent FDA clearance also includes use in the S1 body of the sacrum and pediatric deformity.
Laura Francis, Chief Executive Officer, said, “Given the surgeon enthusiasm around the smaller diameter implant, we are poised to accelerate the adoption of Granite across the nearly 130,000 annual adult deformity and degenerative spine target procedures.”
Source: SI-BONE, Inc.
SI-BONE announced the first cases completed with the smaller, 9.5 mm diameter iFuse Bedrock Granite Implant (Granite 9.5). When placed across the SI joint, the Granite implant provides sacroiliac fusion and spinopelvic fixation as a foundational element for multi-segment spinal fusion.
This milestone for Granite 9.5 comes after FDA 510(k)...
SI-BONE announced the first cases completed with the smaller, 9.5 mm diameter iFuse Bedrock Granite Implant (Granite 9.5). When placed across the SI joint, the Granite implant provides sacroiliac fusion and spinopelvic fixation as a foundational element for multi-segment spinal fusion.
This milestone for Granite 9.5 comes after FDA 510(k) marketing clearance in January 2024. The iFuse Bedrock Granite Implant System was awarded a Breakthrough Device Designation by FDA and a New Technology Add-on Payment by the Centers for Medicare and Medicaid Services. Granite 9.5 may be more suitable for patients with smaller anatomy and may allow easier placement of stacked implants in the sacroalar-iliac trajectory. Moreover, the recent FDA clearance also includes use in the S1 body of the sacrum and pediatric deformity.
Laura Francis, Chief Executive Officer, said, “Given the surgeon enthusiasm around the smaller diameter implant, we are poised to accelerate the adoption of Granite across the nearly 130,000 annual adult deformity and degenerative spine target procedures.”
Source: SI-BONE, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.