Copy to clipboard
Copy to clipboard 
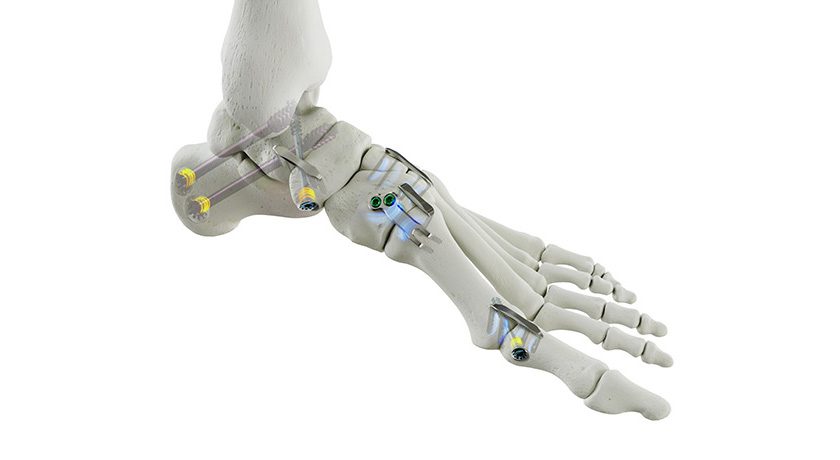
Anika Therapeutics’ Integrity Implant System is now in full market release in the U.S. Integrity comprising a hyaluronic acid-based implant with bone and tendon fixation components and single use arthroscopic delivery instruments, is designed to protect an injured tendon and promote healing in rotator cuff repair and other tendon procedures. The system has been in limited release since November 2023, following FDA 510(k) marketing clearance earlier that year.
“We are proud to have Anika’s new Integrity Implant System now in full market release as we continue to build out our HA-based regenerative product portfolio,” said Cheryl R. Blanchard, Ph.D., Anika’s President and CEO. “The feedback we’ve received during the limited market release highlights the strong differentiation of Integrity compared to existing collagen patches, particularly with the strength and increased regenerative capacity of the implant, and the ease of use of the all-arthroscopic system. Integrity, initially designed to augment rotator cuff repairs, has been clinically used across a wide range of applications including the Achilles tendon and other foot and ankle repairs. We are now seeing strong market pull from surgeons using our Integrity system and we believe it is truly a game changer for surgeons and their patients, quicky becoming the new standard for treating rotator cuff repairs and other tendon repair applications.”
Bench-top testing shows that Integrity provides nearly 50% higher tensile strength, over three times greater suture retention strength, and over three times more tear resistance when hydrated compared to the market-leading collagen implant. Additionally, in a head-to-head preclinical study also comparing Integrity to the market-leading collagen implant, new collagenous tissue infiltration forming a new network of tendon tissue had occurred within the resorbing Integrity structure at 26 weeks, resulting in Integrity demonstrating greater regenerative capacity and the repaired tendon being 3 times greater in thickness.
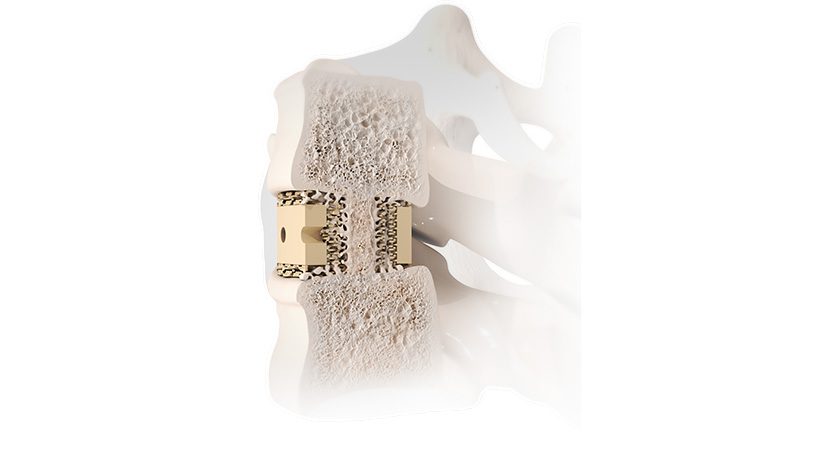
The scaffold component of the Integrity system is a porous, knitted, flexible construct combining Anika’s proprietary Hyaff material, a solid form of HA that supports tissue regeneration and resorbs over time, reinforced with non-resorbable polyethylene terephthalate. Integrity is inherently strong and can be confidently manipulated arthroscopically, which offers a truly unique and differentiated solution for surgeons to treat a variety of tendon repairs. Integrity can be affixed using PEEK bone staples, resorbable PLGA soft tissue tendon tacks or suture fixation, as desired, at the site of the rotator cuff repair.
Source: Anika
Anika Therapeutics' Integrity Implant System is now in full market release in the U.S. Integrity comprising a hyaluronic acid-based implant with bone and tendon fixation components and single use arthroscopic delivery instruments, is designed to protect an injured tendon and promote healing in rotator cuff repair and other tendon procedures. The...
Anika Therapeutics’ Integrity Implant System is now in full market release in the U.S. Integrity comprising a hyaluronic acid-based implant with bone and tendon fixation components and single use arthroscopic delivery instruments, is designed to protect an injured tendon and promote healing in rotator cuff repair and other tendon procedures. The system has been in limited release since November 2023, following FDA 510(k) marketing clearance earlier that year.
“We are proud to have Anika’s new Integrity Implant System now in full market release as we continue to build out our HA-based regenerative product portfolio,” said Cheryl R. Blanchard, Ph.D., Anika’s President and CEO. “The feedback we’ve received during the limited market release highlights the strong differentiation of Integrity compared to existing collagen patches, particularly with the strength and increased regenerative capacity of the implant, and the ease of use of the all-arthroscopic system. Integrity, initially designed to augment rotator cuff repairs, has been clinically used across a wide range of applications including the Achilles tendon and other foot and ankle repairs. We are now seeing strong market pull from surgeons using our Integrity system and we believe it is truly a game changer for surgeons and their patients, quicky becoming the new standard for treating rotator cuff repairs and other tendon repair applications.”
Bench-top testing shows that Integrity provides nearly 50% higher tensile strength, over three times greater suture retention strength, and over three times more tear resistance when hydrated compared to the market-leading collagen implant. Additionally, in a head-to-head preclinical study also comparing Integrity to the market-leading collagen implant, new collagenous tissue infiltration forming a new network of tendon tissue had occurred within the resorbing Integrity structure at 26 weeks, resulting in Integrity demonstrating greater regenerative capacity and the repaired tendon being 3 times greater in thickness.
The scaffold component of the Integrity system is a porous, knitted, flexible construct combining Anika’s proprietary Hyaff material, a solid form of HA that supports tissue regeneration and resorbs over time, reinforced with non-resorbable polyethylene terephthalate. Integrity is inherently strong and can be confidently manipulated arthroscopically, which offers a truly unique and differentiated solution for surgeons to treat a variety of tendon repairs. Integrity can be affixed using PEEK bone staples, resorbable PLGA soft tissue tendon tacks or suture fixation, as desired, at the site of the rotator cuff repair.
Source: Anika

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.