Copy to clipboard
Copy to clipboard 
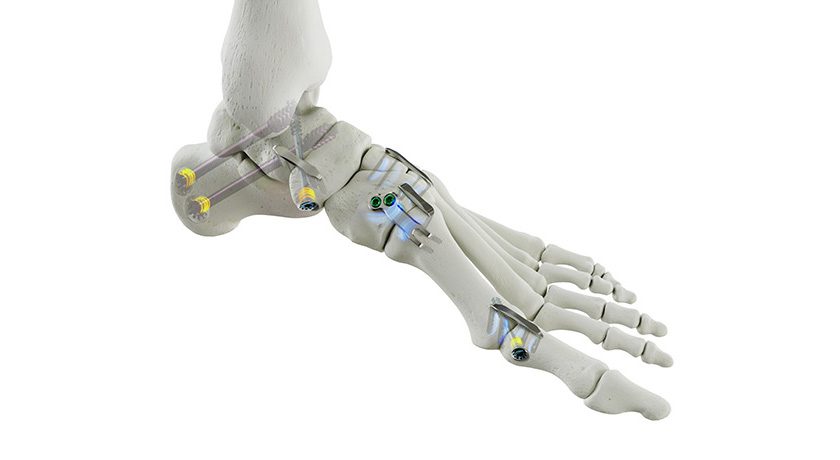
Exactech received FDA 510(k) clearance to market the Truliant Porous Tibial Tray, a 3D tibial knee implant.
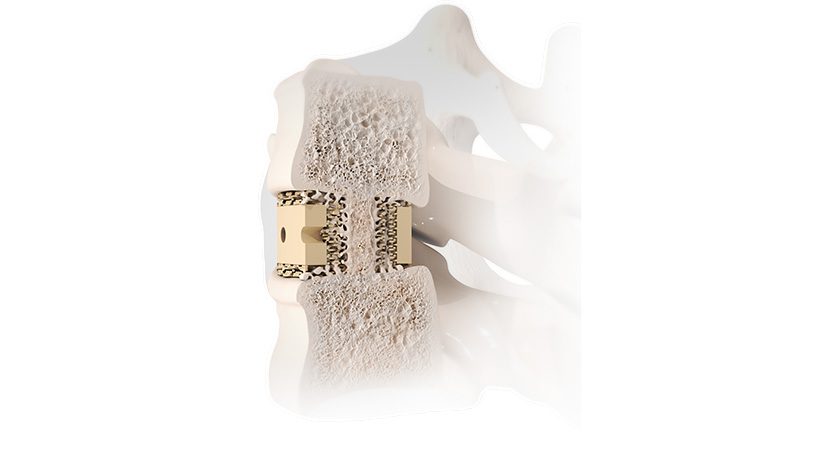
The Truliant Porous Tibial Tray leverages additive manufacturing technology to create a porous structure designed to mimic the structure of cancellous bone. This design aims to facilitate both initial and biological fixation, designed to accommodate patients’ active lifestyles.
Exactech’s Truliant Porous laser-printed 3D tibial tray features peripherally placed tibial pegs, a dual v-channeled keel and optional cancellous bone screws designed to increase initial rotational stability and allow an increased bone-implant interface.
Exactech Chief Marketing Officer and Senior Vice President of Large Joints Adam Hayden said, “By expanding our porous offerings with the laser-printed tray, knee surgeons will have access to additional sizing and fixation options for the personalization of their patients’ total knee replacement procedures, improving upon Exactech’s already successful cementless knee.”
Source: Exactech
Exactech received FDA 510(k) clearance to market the Truliant Porous Tibial Tray, a 3D tibial knee implant.
The Truliant Porous Tibial Tray leverages additive manufacturing technology to create a porous structure designed to mimic the structure of cancellous bone. This design aims to facilitate both initial and biological fixation, designed to...
Exactech received FDA 510(k) clearance to market the Truliant Porous Tibial Tray, a 3D tibial knee implant.
The Truliant Porous Tibial Tray leverages additive manufacturing technology to create a porous structure designed to mimic the structure of cancellous bone. This design aims to facilitate both initial and biological fixation, designed to accommodate patients’ active lifestyles.
Exactech’s Truliant Porous laser-printed 3D tibial tray features peripherally placed tibial pegs, a dual v-channeled keel and optional cancellous bone screws designed to increase initial rotational stability and allow an increased bone-implant interface.
Exactech Chief Marketing Officer and Senior Vice President of Large Joints Adam Hayden said, “By expanding our porous offerings with the laser-printed tray, knee surgeons will have access to additional sizing and fixation options for the personalization of their patients’ total knee replacement procedures, improving upon Exactech’s already successful cementless knee.”
Source: Exactech

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.