
 Copy to clipboard
Copy to clipboard 
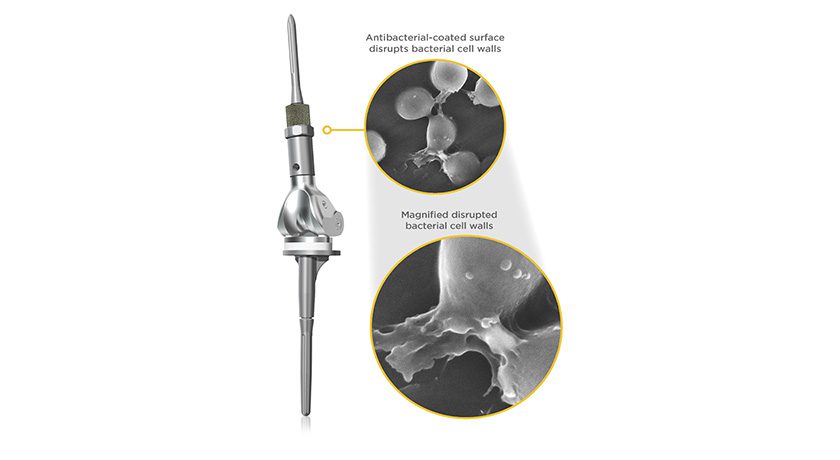
Onkos Surgical’s NanoCept Antibacterial Technology has been used successfully in multiple surgical procedures. NanoCept is a novel antibacterial coating applied to orthopedic implants to help address intraoperative bacterial contamination.
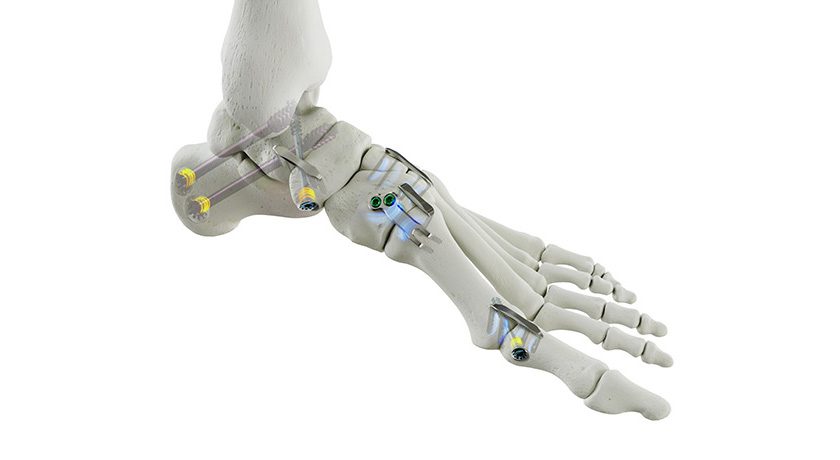
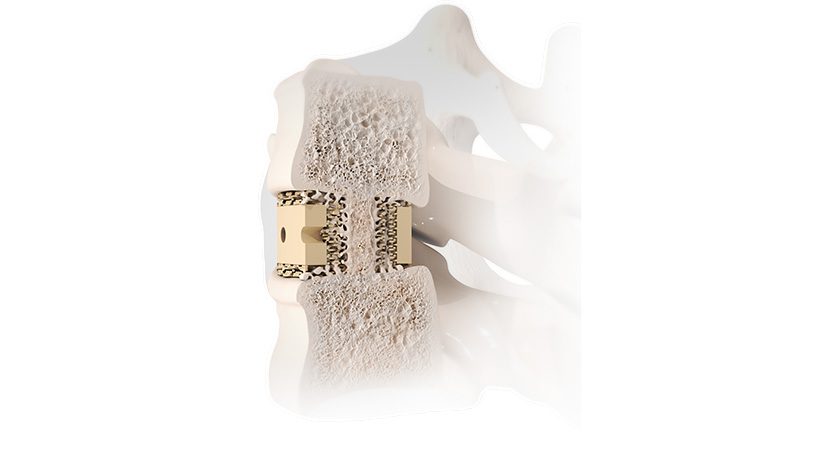
Onkos’ NanoCept Antibacterial Technologyreceived FDA’s De Novo market authorization in 2024. Data submitted to support the De Novo application showed that NanoCept has up to a 99.999% (5-log reduction) kill rate in standardized in vitro testing of bacteria that are commonly found in the operating room environment. The initial FDA authorization for NanoCept was for the Company’s ELEOS Limb Salvage System, which is a modular total joint system used for patients with bone loss.
“The successful completion of these initial surgeries is a key achievement and significant milestone in our strategy to deploy the NanoCept Technology across our portfolio,” said Patrick Treacy, Onkos CEO and Co-founder. “We believe NanoCept is a platform technology that will substantially extend our reach and capability in broad orthopaedic markets, and we are actively working to expand regulatory clearances beyond ELEOS to include implants used in orthopaedic reconstruction due to tumor, trauma and other orthopaedic revision procedures.”
Source: Onkos Surgical
Onkos Surgical's NanoCept Antibacterial Technology has been used successfully in multiple surgical procedures. NanoCept is a novel antibacterial coating applied to orthopedic implants to help address intraoperative bacterial contamination.
Onkos' NanoCept Antibacterial Technologyreceived FDA's De Novo market authorization in 2024. Data...
Onkos Surgical’s NanoCept Antibacterial Technology has been used successfully in multiple surgical procedures. NanoCept is a novel antibacterial coating applied to orthopedic implants to help address intraoperative bacterial contamination.
Onkos’ NanoCept Antibacterial Technologyreceived FDA’s De Novo market authorization in 2024. Data submitted to support the De Novo application showed that NanoCept has up to a 99.999% (5-log reduction) kill rate in standardized in vitro testing of bacteria that are commonly found in the operating room environment. The initial FDA authorization for NanoCept was for the Company’s ELEOS Limb Salvage System, which is a modular total joint system used for patients with bone loss.
“The successful completion of these initial surgeries is a key achievement and significant milestone in our strategy to deploy the NanoCept Technology across our portfolio,” said Patrick Treacy, Onkos CEO and Co-founder. “We believe NanoCept is a platform technology that will substantially extend our reach and capability in broad orthopaedic markets, and we are actively working to expand regulatory clearances beyond ELEOS to include implants used in orthopaedic reconstruction due to tumor, trauma and other orthopaedic revision procedures.”
Source: Onkos Surgical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.