Copy to clipboard
Copy to clipboard 
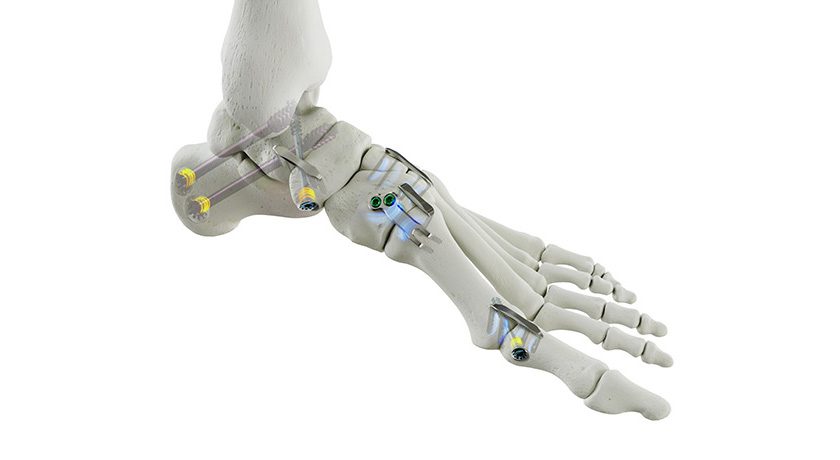
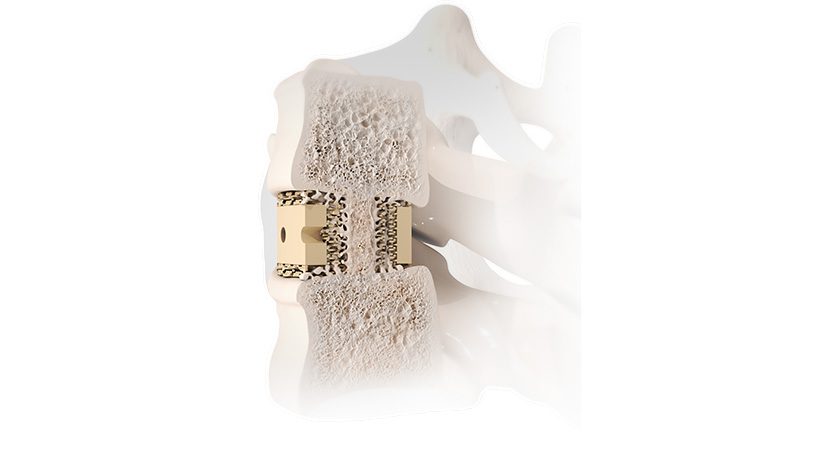
Orthofix Medical received FDA 510(k) clearance and approval under the CE Mark for the TrueLok Elevate Transverse Bone Transport (TBT) System.
TrueLok Elevate provides a limb preservation treatment option for addressing bony or soft tissue deformities and defects such as diabetic foot ulcers and nonhealing or deep tissue wounds. While the TBT procedure has previously been reported, TrueLok Elevate is the first dedicated system for TBT to receive FDA clearance. The product is currently available in a limited market release in the U.S. and select international markets.
The system represents the latest addition to Orthofix’s flagship product line, the TrueLok family of multiplanar external fixators, including the TrueLok EVO and TL-HEX ring fixation systems.
“The introduction of the TrueLok Elevate system is a pivotal milestone in demonstrating our commitment to leading growth in the limb reconstruction market,” said Patrick Fisher, President of Global Orthopedics for Orthofix. “Within our orthopedics business, we are focusing on providing innovative solutions that spans four pillars: Limb Preservation, Extremity Deformity Correction, Limb Lengthening, and Complex Fracture Management to aid surgeons in managing patients with complex limb reconstruction needs.”
Source: Orthofix
Orthofix Medical received FDA 510(k) clearance and approval under the CE Mark for the TrueLok Elevate Transverse Bone Transport (TBT) System.
TrueLok Elevate provides a limb preservation treatment option for addressing bony or soft tissue deformities and defects such as diabetic foot ulcers and nonhealing or deep tissue wounds. While the TBT...
Orthofix Medical received FDA 510(k) clearance and approval under the CE Mark for the TrueLok Elevate Transverse Bone Transport (TBT) System.
TrueLok Elevate provides a limb preservation treatment option for addressing bony or soft tissue deformities and defects such as diabetic foot ulcers and nonhealing or deep tissue wounds. While the TBT procedure has previously been reported, TrueLok Elevate is the first dedicated system for TBT to receive FDA clearance. The product is currently available in a limited market release in the U.S. and select international markets.
The system represents the latest addition to Orthofix’s flagship product line, the TrueLok family of multiplanar external fixators, including the TrueLok EVO and TL-HEX ring fixation systems.
“The introduction of the TrueLok Elevate system is a pivotal milestone in demonstrating our commitment to leading growth in the limb reconstruction market,” said Patrick Fisher, President of Global Orthopedics for Orthofix. “Within our orthopedics business, we are focusing on providing innovative solutions that spans four pillars: Limb Preservation, Extremity Deformity Correction, Limb Lengthening, and Complex Fracture Management to aid surgeons in managing patients with complex limb reconstruction needs.”
Source: Orthofix

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.