Copy to clipboard
Copy to clipboard 
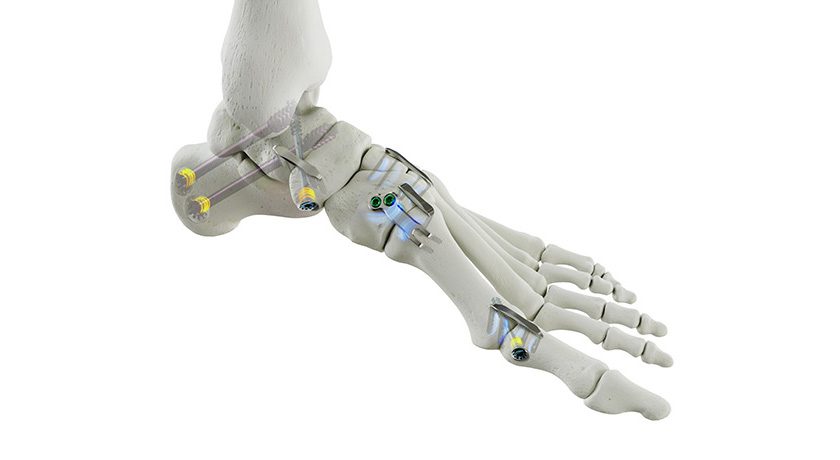
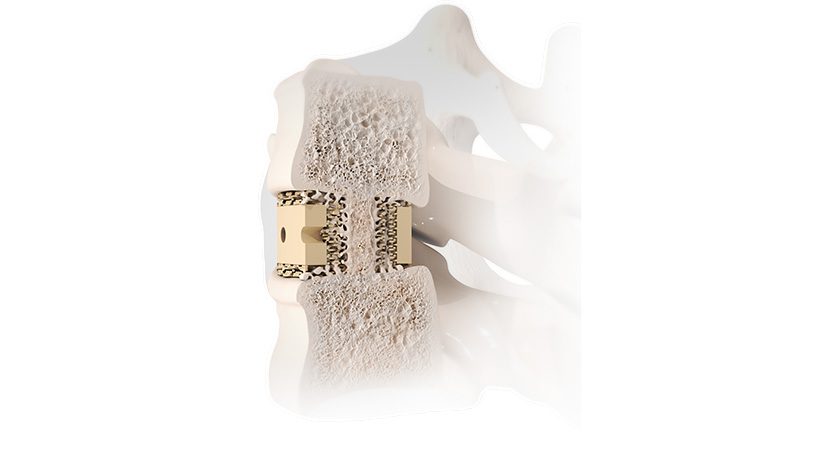
FDA provided clearance of an expanded indication for Tenon Medical’s Catamaran Sacroiliac (SI) Joint Fusion System for use in augmenting thoracolumbar fusion. With this approval, the Catamaran is now indicated to treat the SI joint as either a stand-alone treatment or to augment a spinal fusion.
Catamaran received initial clearance in 2018, when it was noted as the first patented single-implant SIJ fusion system to employ a posterior approach for minimal tissue disruption.
“Recognition from the FDA of Catamaran’s use to augment spinal fusion has been a long-term goal of Tenon during the development of the Catamaran SI Joint Fusion System,” said Steve Foster, Chief Executive Officer of Tenon Medical. “As our clinical experience grows and the data from our MAINSAIL clinical trial emerges, it is clear that we achieve an authentic fusion of the SI Joint in a safe, reliable and efficient manner. We believe this can be an important tool in the complex spine surgeon arsenal to support the base of a multi-level fusion.”
Source: Tenon Medical, Inc.
FDA provided clearance of an expanded indication for Tenon Medical's Catamaran Sacroiliac (SI) Joint Fusion System for use in augmenting thoracolumbar fusion. With this approval, the Catamaran is now indicated to treat the SI joint as either a stand-alone treatment or to augment a spinal fusion.
Catamaran received initial clearance in...
FDA provided clearance of an expanded indication for Tenon Medical’s Catamaran Sacroiliac (SI) Joint Fusion System for use in augmenting thoracolumbar fusion. With this approval, the Catamaran is now indicated to treat the SI joint as either a stand-alone treatment or to augment a spinal fusion.
Catamaran received initial clearance in 2018, when it was noted as the first patented single-implant SIJ fusion system to employ a posterior approach for minimal tissue disruption.
“Recognition from the FDA of Catamaran’s use to augment spinal fusion has been a long-term goal of Tenon during the development of the Catamaran SI Joint Fusion System,” said Steve Foster, Chief Executive Officer of Tenon Medical. “As our clinical experience grows and the data from our MAINSAIL clinical trial emerges, it is clear that we achieve an authentic fusion of the SI Joint in a safe, reliable and efficient manner. We believe this can be an important tool in the complex spine surgeon arsenal to support the base of a multi-level fusion.”
Source: Tenon Medical, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.