
 Copy to clipboard
Copy to clipboard 
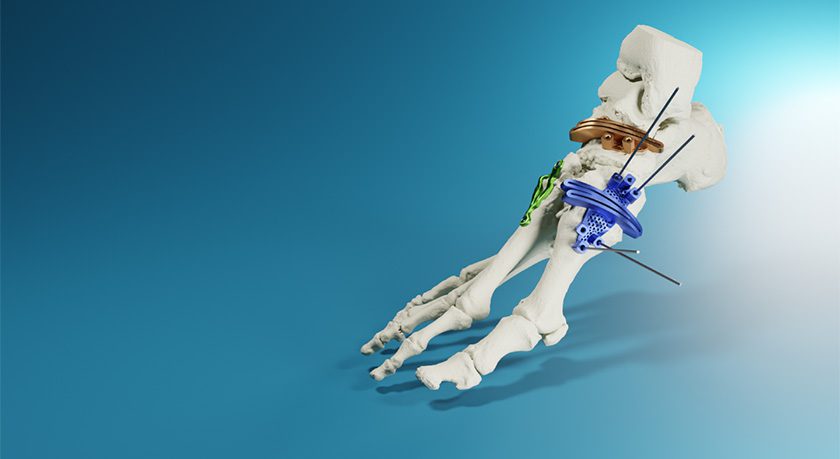
MedCAD was granted FDA 510(k) clearance to market its AccuStride Foot and Ankle System. Coupled with the MedCAD’s proprietary software, the devices will enable foot and ankle surgeons to correct multiple related pathologies in a single procedure.
The patent-pending AccuStride System marks the company’s first offerings for lower extremities; MedCAD is also a manufacturer of patient-specific, custom-designed cranial and maxillofacial reconstruction implants, including virtual planning and surgical devices.
AccuStride is designed to improve proximal phalanx and metatarsal arthroplasty outcomes, and to provide custom solutions for Lapidus revisions and cavus foot corrections. Its components are constructed of UV-curable acrylate polymers or titanium alloy materials and can be delivered in as few as five days after receipt of medical imaging and surgeon design approval. The system is intended for use in patients 12 years and older.
“MedCAD’s newly announced, patient-matched solution is unlike anything else available for surgeons who routinely perform complex or revision cases in the treatment of the entire foot,” said Nancy Hairston, CEO and president of MedCAD. “We have experienced a surge in inquiries and interest from leading foot and ankle orthopedic specialists who are eager to use our ‘whole foot’ solutions for multiple pathologies, and we expect these custom 3D-printed devices to reduce the frequency and duration of surgeries, and to deliver high quality, durable outcomes.”
Source: MedCAD
MedCAD was granted FDA 510(k) clearance to market its AccuStride Foot and Ankle System. Coupled with the MedCAD's proprietary software, the devices will enable foot and ankle surgeons to correct multiple related pathologies in a single procedure.
The patent-pending AccuStride System marks the company's first offerings for lower extremities;...
MedCAD was granted FDA 510(k) clearance to market its AccuStride Foot and Ankle System. Coupled with the MedCAD’s proprietary software, the devices will enable foot and ankle surgeons to correct multiple related pathologies in a single procedure.
The patent-pending AccuStride System marks the company’s first offerings for lower extremities; MedCAD is also a manufacturer of patient-specific, custom-designed cranial and maxillofacial reconstruction implants, including virtual planning and surgical devices.
AccuStride is designed to improve proximal phalanx and metatarsal arthroplasty outcomes, and to provide custom solutions for Lapidus revisions and cavus foot corrections. Its components are constructed of UV-curable acrylate polymers or titanium alloy materials and can be delivered in as few as five days after receipt of medical imaging and surgeon design approval. The system is intended for use in patients 12 years and older.
“MedCAD’s newly announced, patient-matched solution is unlike anything else available for surgeons who routinely perform complex or revision cases in the treatment of the entire foot,” said Nancy Hairston, CEO and president of MedCAD. “We have experienced a surge in inquiries and interest from leading foot and ankle orthopedic specialists who are eager to use our ‘whole foot’ solutions for multiple pathologies, and we expect these custom 3D-printed devices to reduce the frequency and duration of surgeries, and to deliver high quality, durable outcomes.”
Source: MedCAD

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.