Copy to clipboard
Copy to clipboard 
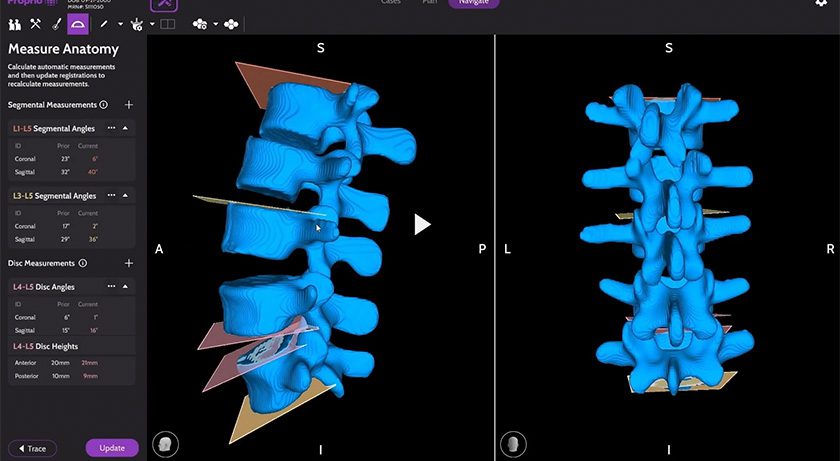
restor3d received FDA 510(k) clearance to market the iTotal Identity CR 3DP Porous Total Knee Replacement. This milestone expands restor3d’s knee replacement technology since the Conformis acquisition, introducing the first cementless offering to the patient-specific implant portfolio. Limited market release is slated for 3Q25.
The Identity 3D-printed cementless system is built on restor3d’s approach to personalized orthopedics, integrating patient-matched design with 3D-printed porous technology. The implant provides an alternative to traditional fixation methods while preserving the benefits of a patient-specific implant. The pre-navigated surgical system enhances efficiency by eliminating intraoperative guesswork, with personalized surgical instruments and implants delivered in a sterile, single-use kit.
Available on the femoral, tibial, and patellar components, the system incorporates restor3d’s TIDAL Technology to deliver optimized osseointegration strength, superior fatigue resistance and load distribution compared to traditional truss-based lattices.
Source: restor3d, Inc.
restor3d received FDA 510(k) clearance to market the iTotal Identity CR 3DP Porous Total Knee Replacement. This milestone expands restor3d’s knee replacement technology since the Conformis acquisition, introducing the first cementless offering to the patient-specific implant portfolio. Limited market release is slated for 3Q25.
The...
restor3d received FDA 510(k) clearance to market the iTotal Identity CR 3DP Porous Total Knee Replacement. This milestone expands restor3d’s knee replacement technology since the Conformis acquisition, introducing the first cementless offering to the patient-specific implant portfolio. Limited market release is slated for 3Q25.
The Identity 3D-printed cementless system is built on restor3d’s approach to personalized orthopedics, integrating patient-matched design with 3D-printed porous technology. The implant provides an alternative to traditional fixation methods while preserving the benefits of a patient-specific implant. The pre-navigated surgical system enhances efficiency by eliminating intraoperative guesswork, with personalized surgical instruments and implants delivered in a sterile, single-use kit.
Available on the femoral, tibial, and patellar components, the system incorporates restor3d’s TIDAL Technology to deliver optimized osseointegration strength, superior fatigue resistance and load distribution compared to traditional truss-based lattices.
Source: restor3d, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.