
 Copy to clipboard
Copy to clipboard 
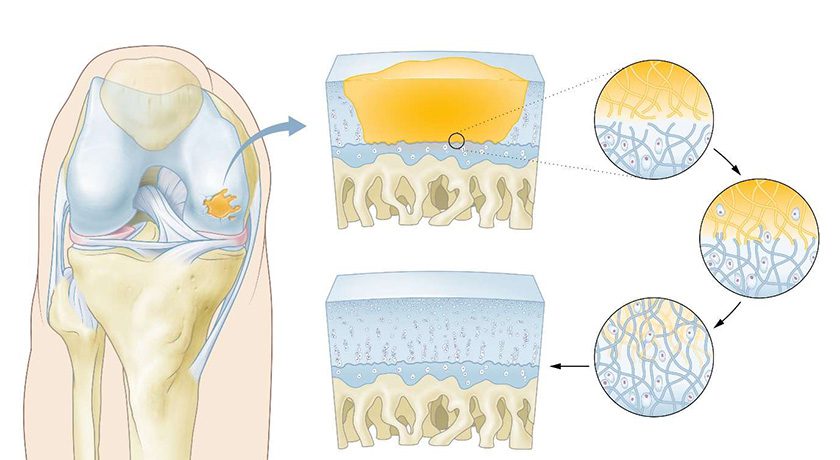
Hy2Care received Investigational Device Exemption (IDE) approval from FDA to initiate its first clinical trial in the United States. This milestone enables the evaluation of Hy2Care’s CartRevive hydrogel implant, designed to enable optimal repair of traumatic cartilage injuries in the knee.
The IDE approval builds on the positive results of Hy2Care’s ongoing European clinical trial and highlights the growing recognition of CartRevive as a minimally invasive solution.
Preparations for the U.S. trial are already underway, with the first patient treatment expected early 2026.
Next steps include approval under the CE Mark, targeted for 2026, with ongoing efforts toward regulatory approval and commercial launch in Europe.
Leo Smit, CEO of Hy2Care, said, “Receiving IDE approval is a significant achievement for Hy2Care. It reflects the strength of our technology, the dedication of our team, and the clinical evidence we have built over the past years. This is not only an important US regulatory step — it is a major step forward in fulfilling our mission of bringing Hy2Care CartRevive hydrogel implant to patients worldwide.”
Co-founder and former patient Dr. Sanne Both added, “We are grateful to the FDA for the smooth approval process. We have been working closely with the agency — first to obtain Breakthrough Device Designation, and now IDE approval. Considering the agency’s high workload, we are truly pleased to have received this approval at this time.”
Source: Hy2Care
Hy2Care received Investigational Device Exemption (IDE) approval from FDA to initiate its first clinical trial in the United States. This milestone enables the evaluation of Hy2Care’s CartRevive hydrogel implant, designed to enable optimal repair of traumatic cartilage injuries in the knee.
The IDE approval builds on the positive results of...
Hy2Care received Investigational Device Exemption (IDE) approval from FDA to initiate its first clinical trial in the United States. This milestone enables the evaluation of Hy2Care’s CartRevive hydrogel implant, designed to enable optimal repair of traumatic cartilage injuries in the knee.
The IDE approval builds on the positive results of Hy2Care’s ongoing European clinical trial and highlights the growing recognition of CartRevive as a minimally invasive solution.
Preparations for the U.S. trial are already underway, with the first patient treatment expected early 2026.
Next steps include approval under the CE Mark, targeted for 2026, with ongoing efforts toward regulatory approval and commercial launch in Europe.
Leo Smit, CEO of Hy2Care, said, “Receiving IDE approval is a significant achievement for Hy2Care. It reflects the strength of our technology, the dedication of our team, and the clinical evidence we have built over the past years. This is not only an important US regulatory step — it is a major step forward in fulfilling our mission of bringing Hy2Care CartRevive hydrogel implant to patients worldwide.”
Co-founder and former patient Dr. Sanne Both added, “We are grateful to the FDA for the smooth approval process. We have been working closely with the agency — first to obtain Breakthrough Device Designation, and now IDE approval. Considering the agency’s high workload, we are truly pleased to have received this approval at this time.”
Source: Hy2Care

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.