
 Copy to clipboard
Copy to clipboard 
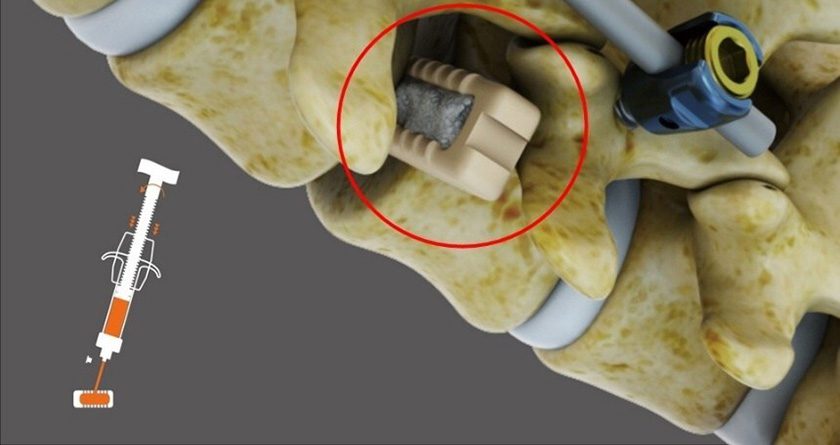
CGBIO received Investigational Device Exemption (IDE) approval from FDA for a U.S. clinical trial of NOVOSIS PUTTY bone graft substitute in spinal fusion procedures.
NOVOSIS PUTTY, previously designated as a Breakthrough Device by FDA, leverages novel technology to address unmet needs in bone regeneration. This milestone marks NOVOSIS PUTTY as the first Korean-developed bio-combined medical device to reach this stage in the U.S., signifying a significant step toward Premarket Approval and subsequent commercialization.
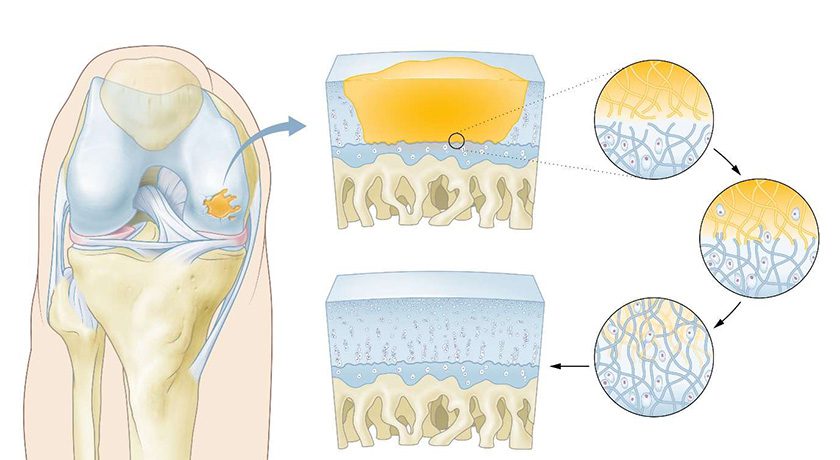
The product features a dual-carrier system utilizing Hydroxyapatite (HA) and Tri-Calcium Phosphate (TCP), combined with CGBIO’s proprietary sustained-release technology, SLOREL, to control the release of recombinant human bone morphogenetic protein-2 (rhBMP-2). This system is engineered to enhance high-density bone formation while minimizing ectopic bone growth, a common adverse effect in earlier rhBMP-2-based products. The safety and efficacy of NOVOSIS PUTTY have been validated in peer-reviewed publications, including the Journal of Clinical Medicine.
Daewoong Pharmaceutical, a strategic partner of CGBIO, manufactures the rhBMP-2 protein used in NOVOSIS PUTTY.
In February 2025, CGBIO and its subsidiary CG MedTech signed a partnership agreement with Johnson & Johnson MedTech for the exclusive supply of NOVOSIS and NOVOSIS TRAUMA products across Korea and other Asian territories. The new IDE approval for NOVOSIS PUTTY is expected to accelerate the global expansion of the entire NOVOSIS product family.
Source: CGBIO
CGBIO received Investigational Device Exemption (IDE) approval from FDA for a U.S. clinical trial of NOVOSIS PUTTY bone graft substitute in spinal fusion procedures.
NOVOSIS PUTTY, previously designated as a Breakthrough Device by FDA, leverages novel technology to address unmet needs in bone regeneration. This milestone marks NOVOSIS...
CGBIO received Investigational Device Exemption (IDE) approval from FDA for a U.S. clinical trial of NOVOSIS PUTTY bone graft substitute in spinal fusion procedures.
NOVOSIS PUTTY, previously designated as a Breakthrough Device by FDA, leverages novel technology to address unmet needs in bone regeneration. This milestone marks NOVOSIS PUTTY as the first Korean-developed bio-combined medical device to reach this stage in the U.S., signifying a significant step toward Premarket Approval and subsequent commercialization.
The product features a dual-carrier system utilizing Hydroxyapatite (HA) and Tri-Calcium Phosphate (TCP), combined with CGBIO’s proprietary sustained-release technology, SLOREL, to control the release of recombinant human bone morphogenetic protein-2 (rhBMP-2). This system is engineered to enhance high-density bone formation while minimizing ectopic bone growth, a common adverse effect in earlier rhBMP-2-based products. The safety and efficacy of NOVOSIS PUTTY have been validated in peer-reviewed publications, including the Journal of Clinical Medicine.
Daewoong Pharmaceutical, a strategic partner of CGBIO, manufactures the rhBMP-2 protein used in NOVOSIS PUTTY.
In February 2025, CGBIO and its subsidiary CG MedTech signed a partnership agreement with Johnson & Johnson MedTech for the exclusive supply of NOVOSIS and NOVOSIS TRAUMA products across Korea and other Asian territories. The new IDE approval for NOVOSIS PUTTY is expected to accelerate the global expansion of the entire NOVOSIS product family.
Source: CGBIO

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.