
 Copy to clipboard
Copy to clipboard 
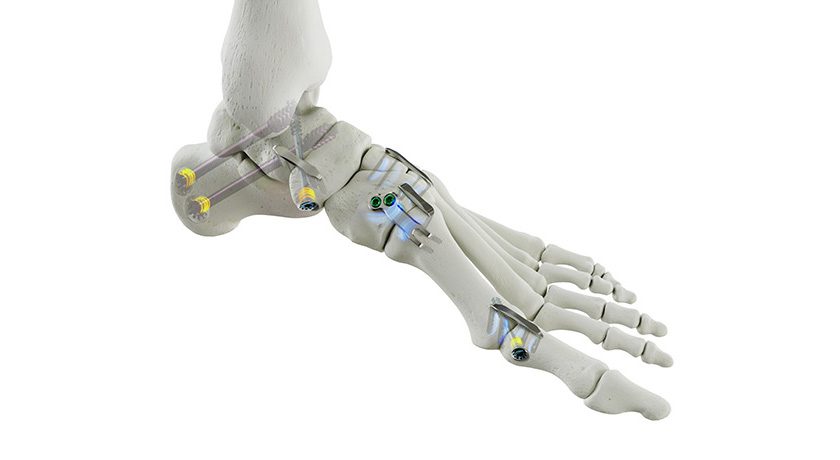
Aurora Spine received FDA 510(k) clearance to market its DEXA SOLO-L™ spinal fusion system. The 3D-printed standalone device was developed as part of what the company claims is the world’s first bone density-matched implant based on Aurora’s DEXA Technology Platform.
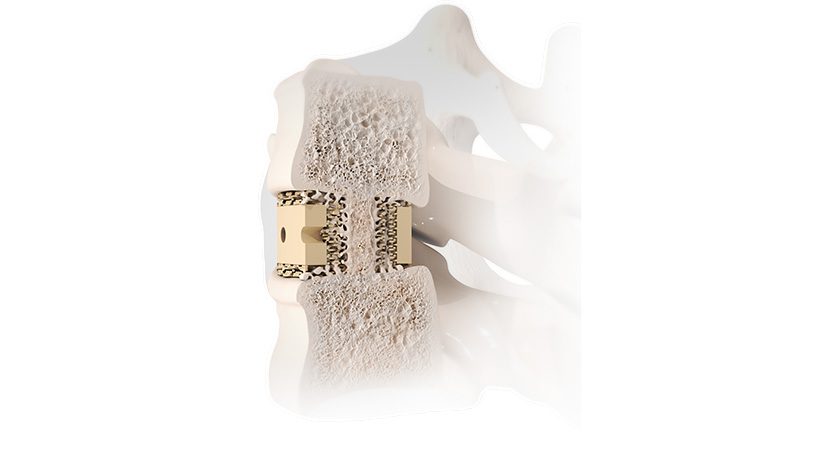
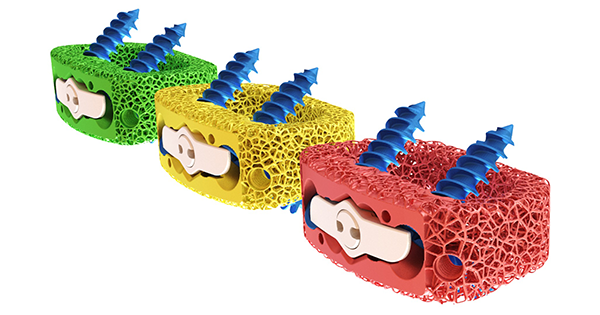
DEXA SOLO-L is intended for use in anterior and lateral lumbar interbody fusion (ALIF & LLIF) procedures and is reportedly the first of its kind device for lumbar spine. It is also the first color-coded, bone-mimicking™ structure implant in the marketplace and is designed to help doctors match the implant to the patents bone quality and density.
Aurora’s DEXA implants match a patient’s bone density and quality to a personalized implant. The DEXA-L product line follows the recently released DEXA-C cervical implant product line.
Mr. Trent Northcutt, President and Chief Executive Officer of Aurora Spine, said, “We are thrilled to receive this new approval for our DEXA SOLO-L device, the world’s first patented and FDA-cleared, color-coded ALIF standalone device. This clearance is an important step to gain new surgeon customers and new sales distribution opportunities nationwide to drive more revenue.”
Source: Aurora Spine
Aurora Spine received FDA 510(k) clearance to market its DEXA SOLO-L™ spinal fusion system. The 3D-printed standalone device was developed as part of what the company claims is the world’s first bone density-matched implant based on Aurora’s DEXA Technology Platform.
DEXA SOLO-L is intended for use in anterior and lateral lumbar interbody fusion...
Aurora Spine received FDA 510(k) clearance to market its DEXA SOLO-L™ spinal fusion system. The 3D-printed standalone device was developed as part of what the company claims is the world’s first bone density-matched implant based on Aurora’s DEXA Technology Platform.
DEXA SOLO-L is intended for use in anterior and lateral lumbar interbody fusion (ALIF & LLIF) procedures and is reportedly the first of its kind device for lumbar spine. It is also the first color-coded, bone-mimicking™ structure implant in the marketplace and is designed to help doctors match the implant to the patents bone quality and density.
Aurora’s DEXA implants match a patient’s bone density and quality to a personalized implant. The DEXA-L product line follows the recently released DEXA-C cervical implant product line.
Mr. Trent Northcutt, President and Chief Executive Officer of Aurora Spine, said, “We are thrilled to receive this new approval for our DEXA SOLO-L device, the world’s first patented and FDA-cleared, color-coded ALIF standalone device. This clearance is an important step to gain new surgeon customers and new sales distribution opportunities nationwide to drive more revenue.”
Source: Aurora Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.