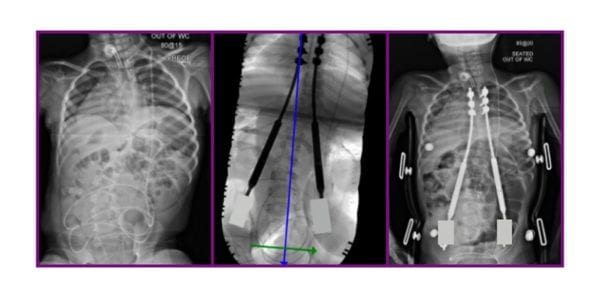
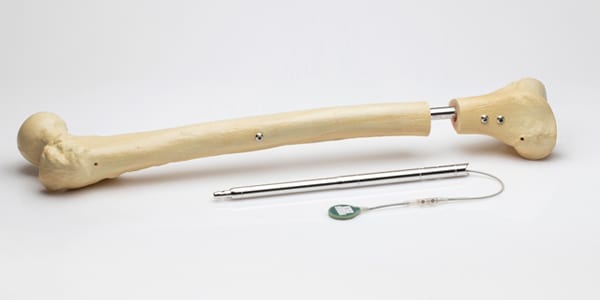
NuVasive Issues Field Safety Notice for MAGEC System
NuVasive voluntarily issued an Urgent Field Safety Notice in the U.K. and Ireland to address concerns identified by The Medicines and Healthcare Products Regulatory Agency over continued use of the MAGEC device for the treatment of scoliosis. Apr 02, 2020