
 Copy to clipboard
Copy to clipboard 
Patient recruitment has commenced for a clinical study, BioPoly Partial Resurfacing Knee Implant IDE. The implant received Breakthrough Device Designation in 2023.
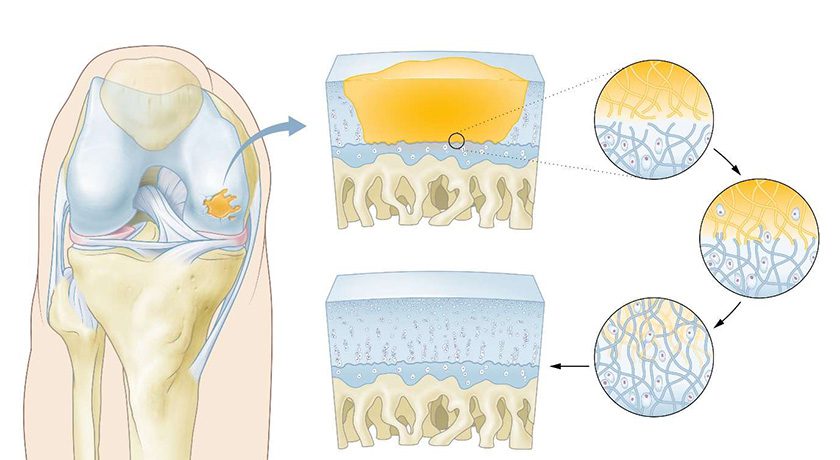
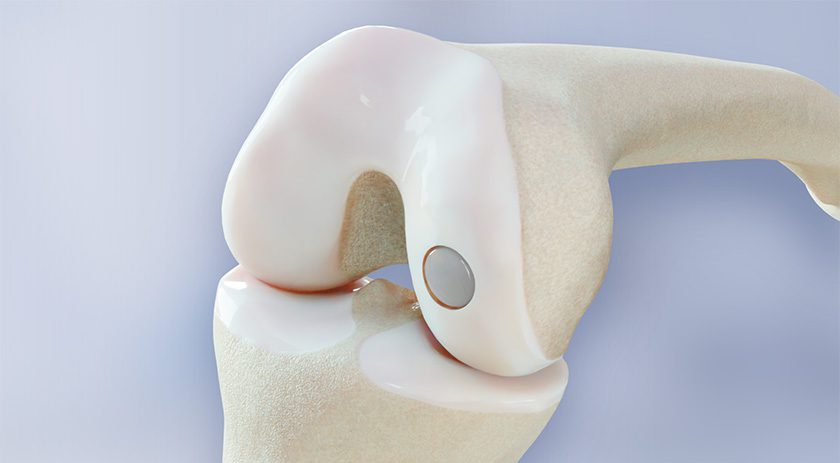
This FDA-approved randomized controlled trial aims to demonstrate the safety and effectiveness of the BioPoly Partial Resurfacing Knee Implant. Specifically designed to address cartilage defects of the distal femur of the knee, this implant is intended for adult patients experiencing persistent knee pain and symptoms who require surgical intervention.
The BioPoly knee implant utilizes a proprietary biomaterial that closely replicates natural cartilage function, providing more natural joint motion. Unlike traditional metal implants, the BioPoly material reduces wear and eliminates issues related to metal sensitivity or allergies. Additionally, compared to biological treatments, BioPoly offers enhanced durability, predictability and immediate structural support without requiring the patient to have robust biology, which many older patients lack.
“Listing our IDE clinical study and patient recruitment through ClinicalTrials.gov underscores BioPoly’s commitment to transparency, scientific rigor, and patient care,” said Dr. Herb Schwartz, CEO of BioPoly LLC. “With our first site up and running, this milestone brings us closer to providing a novel, less invasive treatment option that preserves the natural anatomy of the knee and significantly enhances patient outcomes.”
Long term clinical outcomes from earlier studies conducted in Europe have already demonstrated promising results, reinforcing the potential benefits of this innovative treatment.
This recruitment announcement marks another significant advancement for BioPoly as it continues its mission to improve patient care through innovative orthopedic solutions.
Source: BioPoly LLC
Patient recruitment has commenced for a clinical study, BioPoly Partial Resurfacing Knee Implant IDE. The implant received Breakthrough Device Designation in 2023.
This FDA-approved randomized controlled trial aims to demonstrate the safety and effectiveness of the BioPoly Partial Resurfacing Knee Implant. Specifically designed to address...
Patient recruitment has commenced for a clinical study, BioPoly Partial Resurfacing Knee Implant IDE. The implant received Breakthrough Device Designation in 2023.
This FDA-approved randomized controlled trial aims to demonstrate the safety and effectiveness of the BioPoly Partial Resurfacing Knee Implant. Specifically designed to address cartilage defects of the distal femur of the knee, this implant is intended for adult patients experiencing persistent knee pain and symptoms who require surgical intervention.
The BioPoly knee implant utilizes a proprietary biomaterial that closely replicates natural cartilage function, providing more natural joint motion. Unlike traditional metal implants, the BioPoly material reduces wear and eliminates issues related to metal sensitivity or allergies. Additionally, compared to biological treatments, BioPoly offers enhanced durability, predictability and immediate structural support without requiring the patient to have robust biology, which many older patients lack.
“Listing our IDE clinical study and patient recruitment through ClinicalTrials.gov underscores BioPoly’s commitment to transparency, scientific rigor, and patient care,” said Dr. Herb Schwartz, CEO of BioPoly LLC. “With our first site up and running, this milestone brings us closer to providing a novel, less invasive treatment option that preserves the natural anatomy of the knee and significantly enhances patient outcomes.”
Long term clinical outcomes from earlier studies conducted in Europe have already demonstrated promising results, reinforcing the potential benefits of this innovative treatment.
This recruitment announcement marks another significant advancement for BioPoly as it continues its mission to improve patient care through innovative orthopedic solutions.
Source: BioPoly LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.