
 Copy to clipboard
Copy to clipboard 
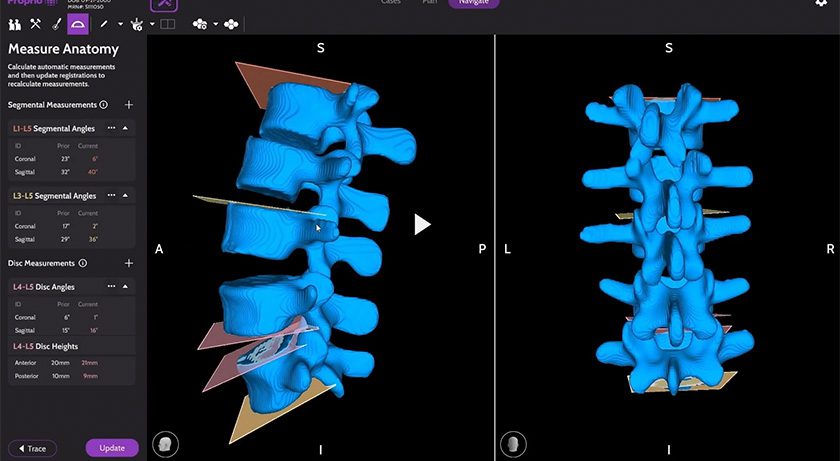
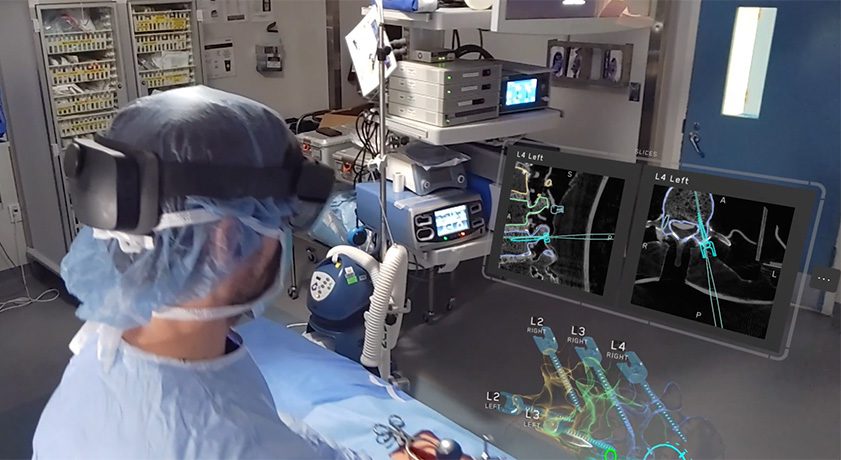
Medivis received FDA 510(k) clearance to market its Spine Navigation platform. This milestone marks an advancement in surgical technology, utilizing AR and AI to empower surgeons with holographic navigation across open and minimally invasive spine procedures. Alongside the FDA clearance, Medivis is announcing the commercial launch of Spine Navigation to U.S. hospitals and ASCs.
“At Medivis, we envision a future where surgical navigation transcends boundaries by integrating imaging data with the operative field. With our recent FDA clearance for Spine Navigation, we are thrilled to equip surgeons with breakthrough technology to visually transform spine surgery and empower them with intuitive image guidance,” added Dr. Osamah Choudhry, CEO of Medivis.
Medivis’ Spine Navigation platform is applicable across neurosurgery and orthopedic surgery, offering:
- Surgical Intelligence: Combining proprietary computer vision algorithms, AI segmentation, real-time data analysis and advanced image processing to deliver precise, context-aware insights throughout the surgical workflow.
- Ergonomic Freedom: Lightweight AR hardware allows surgeons to visualize critical information with unmatched comfort while operating. Hand tracking and voice commands grant complete control of the environment, eliminating attention-shift away from the operative field.
- Seamless Integration: Minimal-footprint workstation paired with intuitive instrumentation allows Medivis’ technology to integrate with spinal hardware and imaging systems across major commercial vendors. It is built for easy deployment in operating rooms, including in ambulatory surgical centers.
Source: Medivis
Medivis received FDA 510(k) clearance to market its Spine Navigation platform. This milestone marks an advancement in surgical technology, utilizing AR and AI to empower surgeons with holographic navigation across open and minimally invasive spine procedures. Alongside the FDA clearance, Medivis is announcing the commercial launch of Spine...
Medivis received FDA 510(k) clearance to market its Spine Navigation platform. This milestone marks an advancement in surgical technology, utilizing AR and AI to empower surgeons with holographic navigation across open and minimally invasive spine procedures. Alongside the FDA clearance, Medivis is announcing the commercial launch of Spine Navigation to U.S. hospitals and ASCs.
“At Medivis, we envision a future where surgical navigation transcends boundaries by integrating imaging data with the operative field. With our recent FDA clearance for Spine Navigation, we are thrilled to equip surgeons with breakthrough technology to visually transform spine surgery and empower them with intuitive image guidance,” added Dr. Osamah Choudhry, CEO of Medivis.
Medivis’ Spine Navigation platform is applicable across neurosurgery and orthopedic surgery, offering:
- Surgical Intelligence: Combining proprietary computer vision algorithms, AI segmentation, real-time data analysis and advanced image processing to deliver precise, context-aware insights throughout the surgical workflow.
- Ergonomic Freedom: Lightweight AR hardware allows surgeons to visualize critical information with unmatched comfort while operating. Hand tracking and voice commands grant complete control of the environment, eliminating attention-shift away from the operative field.
- Seamless Integration: Minimal-footprint workstation paired with intuitive instrumentation allows Medivis’ technology to integrate with spinal hardware and imaging systems across major commercial vendors. It is built for easy deployment in operating rooms, including in ambulatory surgical centers.
Source: Medivis

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.