Copy to clipboard
Copy to clipboard 
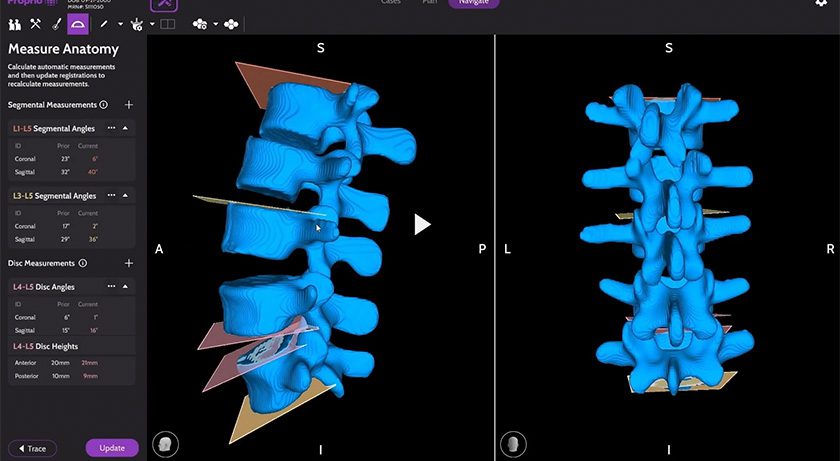
Monogram Technologies was granted FDA 510(k) clearance to market the Monogram mBôs TKA System.
mBôs TKA is designed to support safety, efficiency and accuracy in robotic-assisted total knee arthroplasty. The system is architected to eventually support multiple orthopedic applications beyond knee replacement, positioning it as a platform for future expansion.
Over coming months, Monogram will integrate recent upgrades to the cutting system and other system enhancements into the cleared mBôs TKA System. Upcoming milestones include regulatory and clinical trial preparations to improve future competitiveness and expand to new applications. Further, the company is working to obtain regulatory clearance to initiate clinical trials in India in collaboration with its strategic partner, Shalby, a global multi-specialty hospital chain.
Ben Sexson, Monogram Chief Executive Officer, commented, “Securing clearance for the Monogram mBôs TKA System was a rigorous process due to the complexity and sophistication of the technology. We are particularly excited about the system’s performance and its potential to advance robotics in orthopedic medicine. We believe we will have strong product-market fit and are well-positioned to make a meaningful impact.”
Source: Monogram Technologies Inc.
Monogram Technologies was granted FDA 510(k) clearance to market the Monogram mBôs TKA System.
mBôs TKA is designed to support safety, efficiency and accuracy in robotic-assisted total knee arthroplasty. The system is architected to eventually support multiple orthopedic applications beyond knee replacement, positioning it as a platform for...
Monogram Technologies was granted FDA 510(k) clearance to market the Monogram mBôs TKA System.
mBôs TKA is designed to support safety, efficiency and accuracy in robotic-assisted total knee arthroplasty. The system is architected to eventually support multiple orthopedic applications beyond knee replacement, positioning it as a platform for future expansion.
Over coming months, Monogram will integrate recent upgrades to the cutting system and other system enhancements into the cleared mBôs TKA System. Upcoming milestones include regulatory and clinical trial preparations to improve future competitiveness and expand to new applications. Further, the company is working to obtain regulatory clearance to initiate clinical trials in India in collaboration with its strategic partner, Shalby, a global multi-specialty hospital chain.
Ben Sexson, Monogram Chief Executive Officer, commented, “Securing clearance for the Monogram mBôs TKA System was a rigorous process due to the complexity and sophistication of the technology. We are particularly excited about the system’s performance and its potential to advance robotics in orthopedic medicine. We believe we will have strong product-market fit and are well-positioned to make a meaningful impact.”
Source: Monogram Technologies Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.