
 Copy to clipboard
Copy to clipboard 
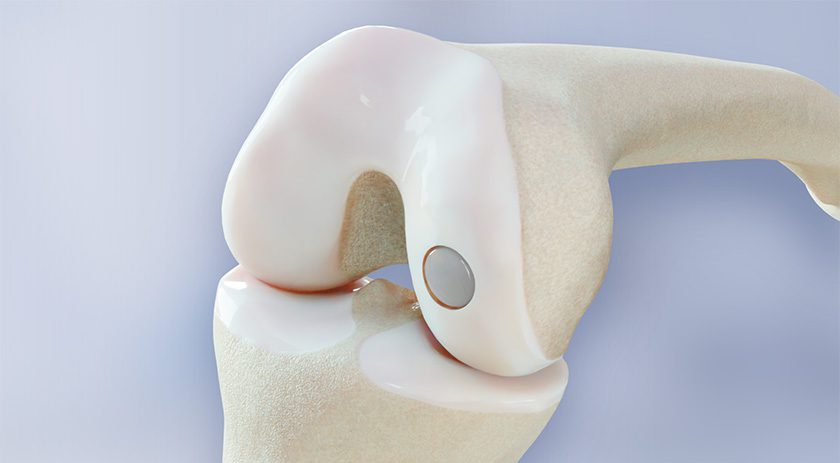
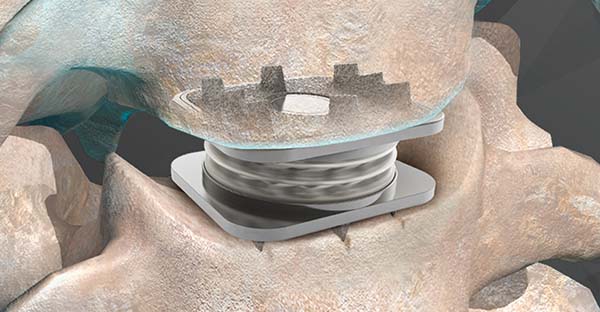
Orthofix Medical announced that more than 60,000 M6-C™ artificial cervical discs have been implanted worldwide. The implant is a next-generation artificial disc designed to mimic the natural motion of a native disc and provide an alternative to cervical fusion. First approved for distribution under the CE Mark in the European Union and other international geographies, the M6-C disc received FDA approval in 2019.
The M6-C disc is comprised of an artificial visco-elastic nucleus and fiber annulus. Like a natural disc, this unique construct allows for shock absorption at the implanted level and provides a controlled range of motion as the spine bends and translates in multiple planes. The M6-C disc is the only such device available in the U.S. with these features.
In August 2021, Orthofix announced the first patient implant in a U.S. Investigational Device Exemption two-level study of the M6-C disc. The study is evaluating patients treated for cervical degenerative disc disease at two contiguous vertebral levels with the M6-C disc compared to anterior cervical discectomy and fusion for symptomatic cervical radiculopathy.
“The M6-C artificial cervical disc is continuing to establish a market-leading position globally with more than 60,000 devices implanted worldwide,” said Orthofix President of Global Spine Kevin Kenny. “We are pleased to see the continued adoption of this state-of-the-art technology as our international success translates into the U.S. market.”
Source: Orthofix
Orthofix Medical announced that more than 60,000 M6-C™ artificial cervical discs have been implanted worldwide. The implant is a next-generation artificial disc designed to mimic the natural motion of a native disc and provide an alternative to cervical fusion. First approved for distribution under the CE Mark in the European Union and other...
Orthofix Medical announced that more than 60,000 M6-C™ artificial cervical discs have been implanted worldwide. The implant is a next-generation artificial disc designed to mimic the natural motion of a native disc and provide an alternative to cervical fusion. First approved for distribution under the CE Mark in the European Union and other international geographies, the M6-C disc received FDA approval in 2019.
The M6-C disc is comprised of an artificial visco-elastic nucleus and fiber annulus. Like a natural disc, this unique construct allows for shock absorption at the implanted level and provides a controlled range of motion as the spine bends and translates in multiple planes. The M6-C disc is the only such device available in the U.S. with these features.
In August 2021, Orthofix announced the first patient implant in a U.S. Investigational Device Exemption two-level study of the M6-C disc. The study is evaluating patients treated for cervical degenerative disc disease at two contiguous vertebral levels with the M6-C disc compared to anterior cervical discectomy and fusion for symptomatic cervical radiculopathy.
“The M6-C artificial cervical disc is continuing to establish a market-leading position globally with more than 60,000 devices implanted worldwide,” said Orthofix President of Global Spine Kevin Kenny. “We are pleased to see the continued adoption of this state-of-the-art technology as our international success translates into the U.S. market.”
Source: Orthofix

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.