Copy to clipboard
Copy to clipboard 
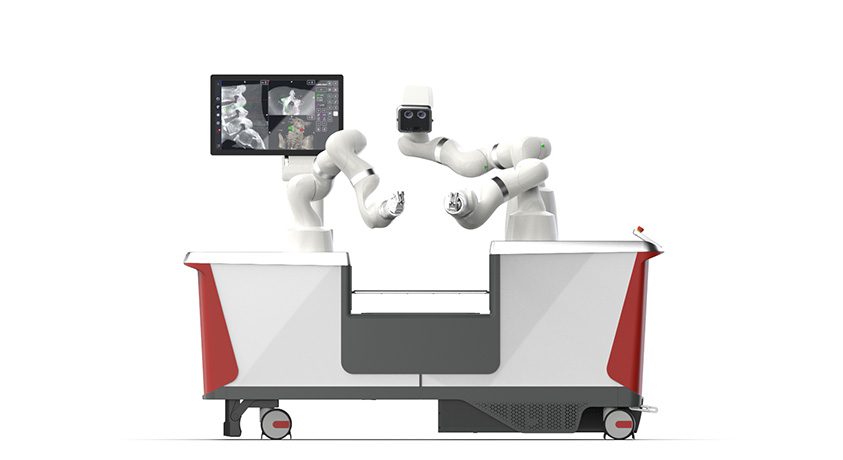
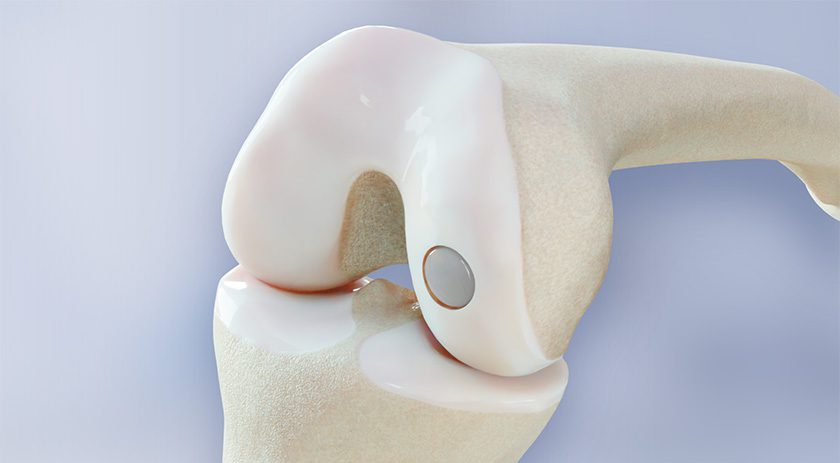
Pixee Medical announced FDA 510(k) clearance to market Knee+ NexSight, the augmented reality (AR) guidance solution for total knee arthroplasty. This next-generation of AR was designed to address the ambulatory surgical centers (ASC) market.
Fully compatible with all primary total knee implants, the system integrates into diverse surgical settings, from high-volume outpatient centers to hospital-based practices.
Surgeons benefit from advanced wearable guidance that enhances intraoperative decision-making and reproducibility, without the need for bulky robotic systems or significant capital investment.
Knee+ NexSight introduces an optional connectivity module that enables secure data exchange with third-party web solutions. This feature unlocks the system’s full potential through secure data sharing capabilities and seamless software updates, positioning surgical teams for enhanced workflow integration and future technological advancements in orthopedic care.
“Knee+ NexSight is not just an evolution of our clinically proven technology, it’s a new approach built around the realities of U.S. outpatient care, informed by the expertise of U.S. surgeons and a proven worldwide track record of our earlier solutions,” explained Sébastien Henry, founder and CEO of Pixee Medical.
Source: Pixee Medical
Pixee Medical announced FDA 510(k) clearance to market Knee+ NexSight, the augmented reality (AR) guidance solution for total knee arthroplasty. This next-generation of AR was designed to address the ambulatory surgical centers (ASC) market.
Fully compatible with all primary total knee implants, the system integrates into diverse surgical...
Pixee Medical announced FDA 510(k) clearance to market Knee+ NexSight, the augmented reality (AR) guidance solution for total knee arthroplasty. This next-generation of AR was designed to address the ambulatory surgical centers (ASC) market.
Fully compatible with all primary total knee implants, the system integrates into diverse surgical settings, from high-volume outpatient centers to hospital-based practices.
Surgeons benefit from advanced wearable guidance that enhances intraoperative decision-making and reproducibility, without the need for bulky robotic systems or significant capital investment.
Knee+ NexSight introduces an optional connectivity module that enables secure data exchange with third-party web solutions. This feature unlocks the system’s full potential through secure data sharing capabilities and seamless software updates, positioning surgical teams for enhanced workflow integration and future technological advancements in orthopedic care.
“Knee+ NexSight is not just an evolution of our clinically proven technology, it’s a new approach built around the realities of U.S. outpatient care, informed by the expertise of U.S. surgeons and a proven worldwide track record of our earlier solutions,” explained Sébastien Henry, founder and CEO of Pixee Medical.
Source: Pixee Medical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.