Copy to clipboard
Copy to clipboard 
Novadip Biosciences announced positive data from its Phase I/II clinical trial evaluating the safety and clinical activity of its investigational product, NVD-003, in patients with severe bone non-union (BNU) of the lower limb following trauma.
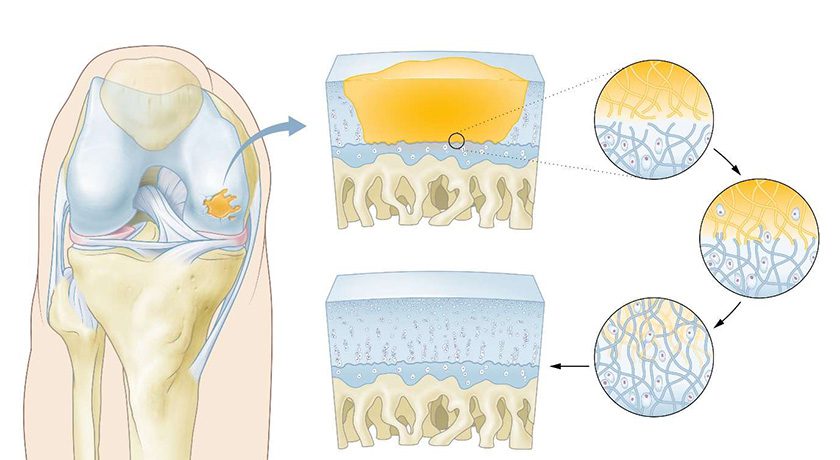
In this study, NVD-003, an autologous tissue-engineered product generated from the patient’s own adipose stem cells, was applied in nine patients with bone non-union of the lower limb who had previously undergone several surgical procedures; in one case, a patient had undergone 14 previous procedures.
Highlights from the clinical study results include:
- No safety issues or implant-associated complications from using the autologous bone tissue-engineered product in patients with severe bone non-healing conditions were observed
- Two-year clinical and radiological follow-up confirmed NVD-003 achieved stable and irreversible bone healing in patients with recalcitrant bone non-union in the lower limb following failure of conventional surgical and bone engraftment treatments
- Rapid bone formation was confirmed at three months post-implantation in all patients
- Clinical healing with weight bearing and walking at six months post-implantation
- 100% success for the NVD-003’s manufacturing capabilities from autologous adipose stem cells
The results demonstrate NVD-003’s ability to reverse severe bone deterioration and to achieve accelerated ossification. A total of eight patients presented clinical healing during the two years of follow-up post-GS. The median and mean time to clinical healing were six months and nine months, respectively. All patients achieved total weight bearing at six months and seven patients were walking normally at two years.
Novadip recently enrolled and treated the first patient in a Phase Ib/IIa clinical trial to study NVD-003 in congenital pseudoarthrosis of the tibia patients between two and eight years of age in the US and EU. The trial will enroll a total of four patients.
Source: Novadip Biosciences
Novadip Biosciences announced positive data from its Phase I/II clinical trial evaluating the safety and clinical activity of its investigational product, NVD-003, in patients with severe bone non-union (BNU) of the lower limb following trauma.
In this study, NVD-003, an autologous tissue-engineered product generated from the patient’s own...
Novadip Biosciences announced positive data from its Phase I/II clinical trial evaluating the safety and clinical activity of its investigational product, NVD-003, in patients with severe bone non-union (BNU) of the lower limb following trauma.
In this study, NVD-003, an autologous tissue-engineered product generated from the patient’s own adipose stem cells, was applied in nine patients with bone non-union of the lower limb who had previously undergone several surgical procedures; in one case, a patient had undergone 14 previous procedures.
Highlights from the clinical study results include:
- No safety issues or implant-associated complications from using the autologous bone tissue-engineered product in patients with severe bone non-healing conditions were observed
- Two-year clinical and radiological follow-up confirmed NVD-003 achieved stable and irreversible bone healing in patients with recalcitrant bone non-union in the lower limb following failure of conventional surgical and bone engraftment treatments
- Rapid bone formation was confirmed at three months post-implantation in all patients
- Clinical healing with weight bearing and walking at six months post-implantation
- 100% success for the NVD-003’s manufacturing capabilities from autologous adipose stem cells
The results demonstrate NVD-003’s ability to reverse severe bone deterioration and to achieve accelerated ossification. A total of eight patients presented clinical healing during the two years of follow-up post-GS. The median and mean time to clinical healing were six months and nine months, respectively. All patients achieved total weight bearing at six months and seven patients were walking normally at two years.
Novadip recently enrolled and treated the first patient in a Phase Ib/IIa clinical trial to study NVD-003 in congenital pseudoarthrosis of the tibia patients between two and eight years of age in the US and EU. The trial will enroll a total of four patients.
Source: Novadip Biosciences

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.