
 Copy to clipboard
Copy to clipboard 
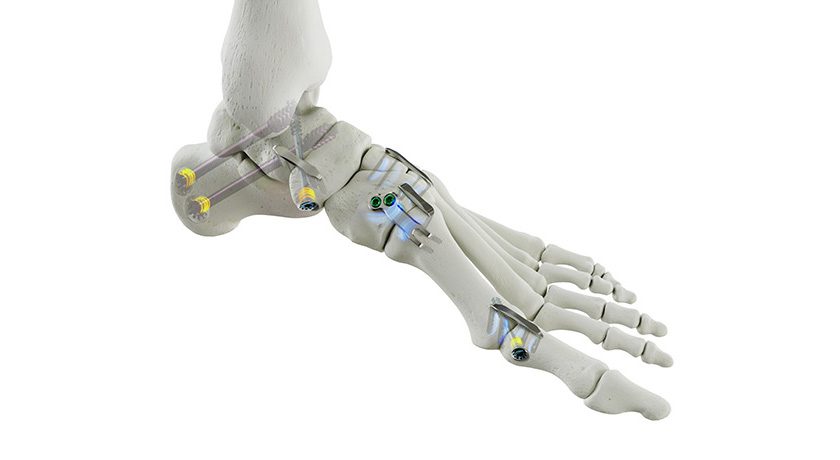
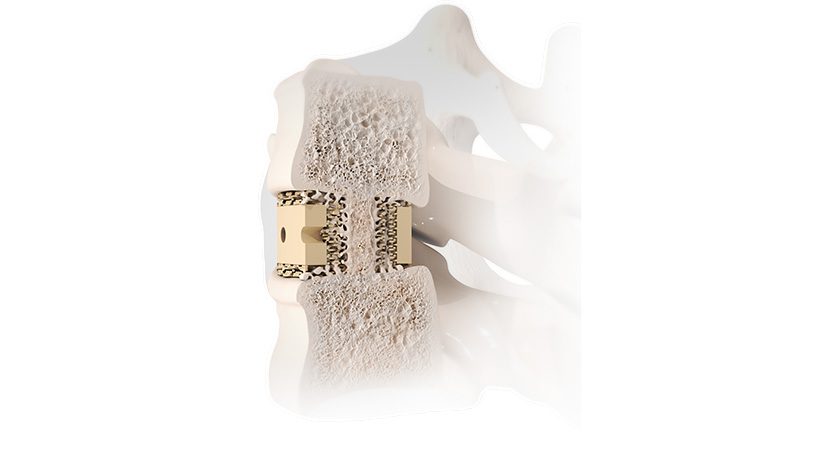
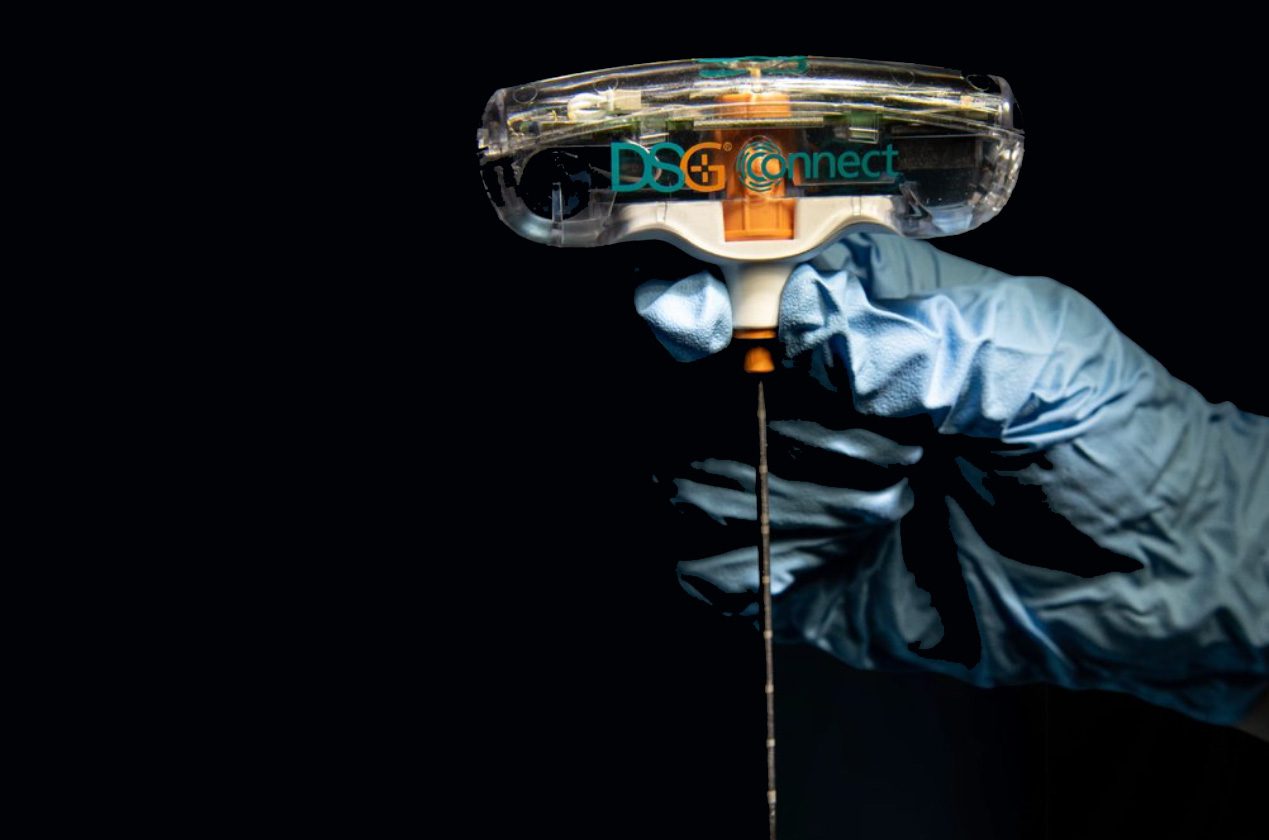
SpineGuard and commercial partner Omnia Medical launched their co-developed PsiFGuard product in the U.S. The smart drilling device is designed to secure the Posterior sacroiliac Fusion (PsiF) surgical procedure. The system received FDA 510(k) marketing clearance in later 2024.
Stéphane Bette, Deputy CEO and co-founder of SpineGuard, stated: “We are particularly excited to collaborate with Omnia Medical to launch PsiFGuard in the market with the support of the clinical experts from our team who recently joined Omnia. PsiFGuard is part of an innovative solution combining our technologies into an integrated kit matching perfectly the practical needs of healthcare providers offering ambulatory sacro-iliac joint fusion procedures to patients. Omnia Medical is the pioneer company in this area and continues to deliver to physicians an unmatched clinical value proposition thanks to the efficacy of our PsiFGuard device in locating the SI joint. For SpineGuard, this new sacroiliac application of our DSG® technology embedded in a well reimbursed fast-growing procedure represents a game-changing opportunity of fast adoption by the market and significant contribution to the growth of our revenue.”
In 2025, SpineGuard continues to focus on sustaining its sales momentum by relying on the introduction of its new products on the market, the Threaded PediGuard for scoliosis correction via anterior approach recently CE marked, and the PsiFGuard device co-developed with Omnia Medical for sacroiliac fusion which recently obtained FDA clearance. The company is also pursuing registration of the whole PediGuard product range in China. Moreover, SpineGuard is working on building strategic partnerships and on strengthening its financial position in continuing to explore various financing options.
Source: SpineGuard
SpineGuard and commercial partner Omnia Medical launched their co-developed PsiFGuard product in the U.S. The smart drilling device is designed to secure the Posterior sacroiliac Fusion (PsiF) surgical procedure. The system received FDA 510(k) marketing clearance in later 2024.
Stéphane Bette, Deputy CEO and co-founder of SpineGuard,...
SpineGuard and commercial partner Omnia Medical launched their co-developed PsiFGuard product in the U.S. The smart drilling device is designed to secure the Posterior sacroiliac Fusion (PsiF) surgical procedure. The system received FDA 510(k) marketing clearance in later 2024.
Stéphane Bette, Deputy CEO and co-founder of SpineGuard, stated: “We are particularly excited to collaborate with Omnia Medical to launch PsiFGuard in the market with the support of the clinical experts from our team who recently joined Omnia. PsiFGuard is part of an innovative solution combining our technologies into an integrated kit matching perfectly the practical needs of healthcare providers offering ambulatory sacro-iliac joint fusion procedures to patients. Omnia Medical is the pioneer company in this area and continues to deliver to physicians an unmatched clinical value proposition thanks to the efficacy of our PsiFGuard device in locating the SI joint. For SpineGuard, this new sacroiliac application of our DSG® technology embedded in a well reimbursed fast-growing procedure represents a game-changing opportunity of fast adoption by the market and significant contribution to the growth of our revenue.”
In 2025, SpineGuard continues to focus on sustaining its sales momentum by relying on the introduction of its new products on the market, the Threaded PediGuard for scoliosis correction via anterior approach recently CE marked, and the PsiFGuard device co-developed with Omnia Medical for sacroiliac fusion which recently obtained FDA clearance. The company is also pursuing registration of the whole PediGuard product range in China. Moreover, SpineGuard is working on building strategic partnerships and on strengthening its financial position in continuing to explore various financing options.
Source: SpineGuard

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.