

 Copy to clipboard
Copy to clipboard 
Sports medicine is such a cool market. Though it claims a smaller share than other orthopedic segments by revenue (about 12% of the total market), we expect it to maintain a buoyant growth rate as younger patients need orthopedic care and older patients seek to remain active for longer. (Pickleball.)
Sports medicine’s high growth rate and penetration in the ASC setting make it an area of strategic focus for the largest players in orthopedics, leading to a highly-consolidated market.
Smaller companies pop up regularly with exciting contributions. Let’s look more closely at some of the products that made the news in 1Q25 and the companies behind them: ABANZA, Arcuro Medical, Atreon Orthopedics and OrthoPreserve.
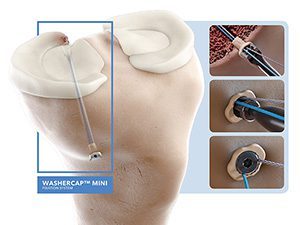 ABANZA Gains FDA 510(k) Clearance for WasherCap Mini Fixation
ABANZA Gains FDA 510(k) Clearance for WasherCap Mini Fixation
WasherCap Mini is the first suture and tape fixation device that offers a knotless, bidirectionally tension-adjustable solution, regardless of bone quality. This device is designed for multiple applications, including meniscal root repair and ACL reconstruction.
Biomechanical testing has shown that WasherCap Mini delivers superior fixation strength and minimal displacement during cyclic loading when compared to conventional devices, like cortical buttons and suture anchors.
ABANZA’s product pipeline includes the WasherCap In Line and the LoopCap, which will support procedures for biceps tenodesis, medial collateral ligament repairs and challenging foot and ankle pathologies.
ABANZA was founded in 2017 in Navarra, Spain.
 Arcuro Medical Gains FDA 510(k) for SuperBall RC
Arcuro Medical Gains FDA 510(k) for SuperBall RC
SuperBall-RC for rotator cuff repair will enter a limited user release in 2Q25, followed by a full launch in 2H25.
Current rotator cuff re-tear rates following surgery can range from 20% to 40% in patients over 50. Rotator cuff augmentation with biologic or biosynthetic grafts can mitigate this risk but can also be technically demanding with current surgical techniques. SuperBall-RC, which is based on Arcuro’s SuperBall technology platform, has also been successfully used in over 5,000 meniscus repairs.
Founded in 2013, Arcuro is headquartered in Misgav, Israel. The company closed a Series A financing round earlier this year for an undisclosed sum. Leadership brings experience from roles at Arthrex, AxioMed, Collagen Solutions, DJO and Nexa Orthopedics.
 Atreon Orthopedics Expands ROTIUM Bioresorbable Wick Indications and Launches BioCharge Autobiologic Matrix
Atreon Orthopedics Expands ROTIUM Bioresorbable Wick Indications and Launches BioCharge Autobiologic Matrix
Atreon received FDA 510(k) clearance to expand the use of ROTIUM Bioresorbable Wick to all tendon repairs, building on its success in rotator cuff procedures.
ROTIUM is a fully resorbable, synthetic-nanofiber scaffold designed to optimize the healing environment, promote tissue remodeling and improve long-term outcomes. Having been used in over 12,000 rotator cuff cases, ROTIUM is now being adopted for a wider range of high-volume tendon repair procedures, including Achilles, triceps, patellar, quadricep, gluteus medius and various applications for the foot and ankle.
Further, the company secured FDA 510(k) clearance and commenced the full U.S. launch of BioCharge Autobiologic Matrix, a bioresorbable synthetic implant designed to address biological failure modes in rotator cuff repair while improving repair integrity and long-term patient outcomes.
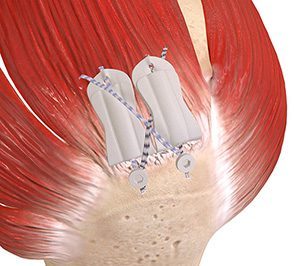 BioCharge is designed as an onlay scaffold to kickstart the patient’s natural healing response, promote cellular activity to encourage native collagen remodeling and reinforce the suture-tendon interface. Atreon’s electrospun nanofiber platform technology offers an alternative to traditional augment devices, which rely on animal-processed collagen or human dermal allografts that can carry patient compatibility risks and a costly manufacturing process.
BioCharge is designed as an onlay scaffold to kickstart the patient’s natural healing response, promote cellular activity to encourage native collagen remodeling and reinforce the suture-tendon interface. Atreon’s electrospun nanofiber platform technology offers an alternative to traditional augment devices, which rely on animal-processed collagen or human dermal allografts that can carry patient compatibility risks and a costly manufacturing process.
Atreon was established in Columbus, Ohio, in 2016. The company raised seed funding of $3.8 million in 2022 and is led by a team that formerly held positions at Cayenne Medical/Zimmer Biomet, DJO, Lipogems, Medtronic and OrthoLogic.
 OrthoPreserve Gains Breakthrough Designation for Meniscus Implant
OrthoPreserve Gains Breakthrough Designation for Meniscus Implant
OrthoPreserve was granted both a Breakthrough Device Designation and Total Product Life Cycle Advisory Program (TAP) enrollment from FDA for Defender, a meniscus replacement. The implant potentially offers an alternative to partial meniscectomy.
The designation covers the use of the therapeutic medical device to treat patients who continue to experience knee pain or impairment following meniscus surgery. Prior to January 2025, orthopedic devices were not part of the TAP program, and Defender is believed to be the first orthopedic device to win TAP enrollment.
OrthoPreserve seeks to launch their pilot clinical trial in 2026, while aiming for potential FDA clearance by 2029.
The company is the “youngest” in this roundup, founded in Atlanta, Georgia in 2021. They have been chosen to pitch at the MedTech Innovator East Coast Pitch Event later this month. We wish them the best!
Sports medicine is such a cool market. Though it claims a smaller share than other orthopedic segments by revenue (about 12% of the total market), we expect it to maintain a buoyant growth rate as younger patients need orthopedic care and older patients seek to remain active for longer. (Pickleball.)
Sports medicine’s high growth rate and...
Sports medicine is such a cool market. Though it claims a smaller share than other orthopedic segments by revenue (about 12% of the total market), we expect it to maintain a buoyant growth rate as younger patients need orthopedic care and older patients seek to remain active for longer. (Pickleball.)
Sports medicine’s high growth rate and penetration in the ASC setting make it an area of strategic focus for the largest players in orthopedics, leading to a highly-consolidated market.
Smaller companies pop up regularly with exciting contributions. Let’s look more closely at some of the products that made the news in 1Q25 and the companies behind them: ABANZA, Arcuro Medical, Atreon Orthopedics and OrthoPreserve.
 ABANZA Gains FDA 510(k) Clearance for WasherCap Mini Fixation
ABANZA Gains FDA 510(k) Clearance for WasherCap Mini Fixation
WasherCap Mini is the first suture and tape fixation device that offers a knotless, bidirectionally tension-adjustable solution, regardless of bone quality. This device is designed for multiple applications, including meniscal root repair and ACL reconstruction.
Biomechanical testing has shown that WasherCap Mini delivers superior fixation strength and minimal displacement during cyclic loading when compared to conventional devices, like cortical buttons and suture anchors.
ABANZA’s product pipeline includes the WasherCap In Line and the LoopCap, which will support procedures for biceps tenodesis, medial collateral ligament repairs and challenging foot and ankle pathologies.
ABANZA was founded in 2017 in Navarra, Spain.
 Arcuro Medical Gains FDA 510(k) for SuperBall RC
Arcuro Medical Gains FDA 510(k) for SuperBall RC
SuperBall-RC for rotator cuff repair will enter a limited user release in 2Q25, followed by a full launch in 2H25.
Current rotator cuff re-tear rates following surgery can range from 20% to 40% in patients over 50. Rotator cuff augmentation with biologic or biosynthetic grafts can mitigate this risk but can also be technically demanding with current surgical techniques. SuperBall-RC, which is based on Arcuro’s SuperBall technology platform, has also been successfully used in over 5,000 meniscus repairs.
Founded in 2013, Arcuro is headquartered in Misgav, Israel. The company closed a Series A financing round earlier this year for an undisclosed sum. Leadership brings experience from roles at Arthrex, AxioMed, Collagen Solutions, DJO and Nexa Orthopedics.
 Atreon Orthopedics Expands ROTIUM Bioresorbable Wick Indications and Launches BioCharge Autobiologic Matrix
Atreon Orthopedics Expands ROTIUM Bioresorbable Wick Indications and Launches BioCharge Autobiologic Matrix
Atreon received FDA 510(k) clearance to expand the use of ROTIUM Bioresorbable Wick to all tendon repairs, building on its success in rotator cuff procedures.
ROTIUM is a fully resorbable, synthetic-nanofiber scaffold designed to optimize the healing environment, promote tissue remodeling and improve long-term outcomes. Having been used in over 12,000 rotator cuff cases, ROTIUM is now being adopted for a wider range of high-volume tendon repair procedures, including Achilles, triceps, patellar, quadricep, gluteus medius and various applications for the foot and ankle.
Further, the company secured FDA 510(k) clearance and commenced the full U.S. launch of BioCharge Autobiologic Matrix, a bioresorbable synthetic implant designed to address biological failure modes in rotator cuff repair while improving repair integrity and long-term patient outcomes.
 BioCharge is designed as an onlay scaffold to kickstart the patient’s natural healing response, promote cellular activity to encourage native collagen remodeling and reinforce the suture-tendon interface. Atreon’s electrospun nanofiber platform technology offers an alternative to traditional augment devices, which rely on animal-processed collagen or human dermal allografts that can carry patient compatibility risks and a costly manufacturing process.
BioCharge is designed as an onlay scaffold to kickstart the patient’s natural healing response, promote cellular activity to encourage native collagen remodeling and reinforce the suture-tendon interface. Atreon’s electrospun nanofiber platform technology offers an alternative to traditional augment devices, which rely on animal-processed collagen or human dermal allografts that can carry patient compatibility risks and a costly manufacturing process.
Atreon was established in Columbus, Ohio, in 2016. The company raised seed funding of $3.8 million in 2022 and is led by a team that formerly held positions at Cayenne Medical/Zimmer Biomet, DJO, Lipogems, Medtronic and OrthoLogic.
 OrthoPreserve Gains Breakthrough Designation for Meniscus Implant
OrthoPreserve Gains Breakthrough Designation for Meniscus Implant
OrthoPreserve was granted both a Breakthrough Device Designation and Total Product Life Cycle Advisory Program (TAP) enrollment from FDA for Defender, a meniscus replacement. The implant potentially offers an alternative to partial meniscectomy.
The designation covers the use of the therapeutic medical device to treat patients who continue to experience knee pain or impairment following meniscus surgery. Prior to January 2025, orthopedic devices were not part of the TAP program, and Defender is believed to be the first orthopedic device to win TAP enrollment.
OrthoPreserve seeks to launch their pilot clinical trial in 2026, while aiming for potential FDA clearance by 2029.
The company is the “youngest” in this roundup, founded in Atlanta, Georgia in 2021. They have been chosen to pitch at the MedTech Innovator East Coast Pitch Event later this month. We wish them the best!

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







