
 Copy to clipboard
Copy to clipboard 
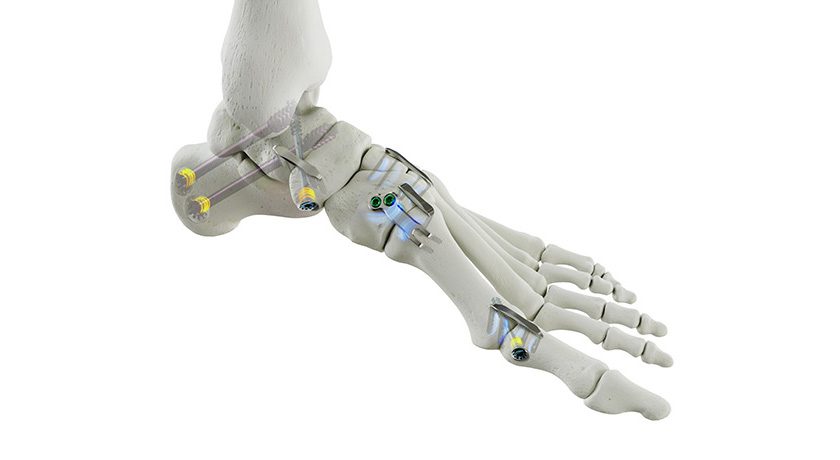
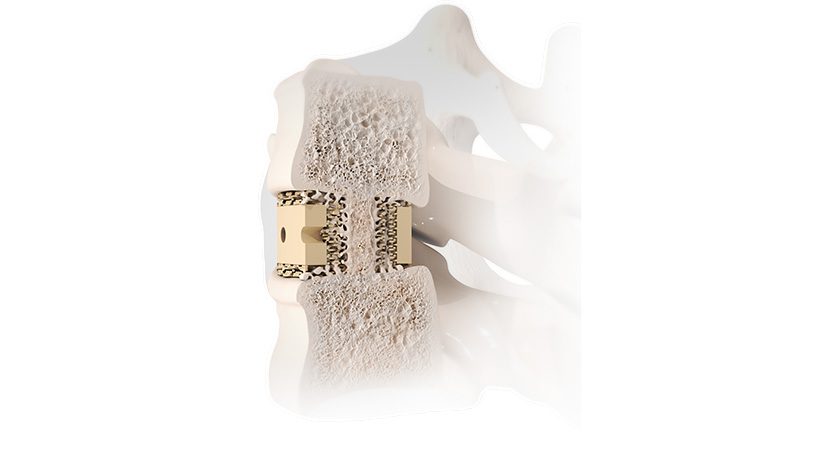
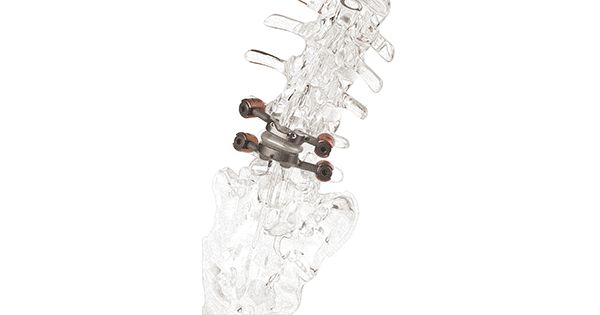
Premia Spine announced clinical data demonstrating that the TOPS Spinal Arthroplasty System provides significant health system and societal benefit vs. transforaminal lumbar interbody fusion (TLIF). Reported to be the first and only facet joint replacement system for the lumbar spine, TOPS was developed to provide lasting mobility, stability and durability after decompression for patients with lumbar spinal stenosis and degenerative spondylolisthesis.
The study explored the cost-effectiveness and economic analysis of TOPS versus TLIF for patients with spondylolisthesis and spinal stenosis. Patients were randomly assigned to either TOPS or TLIF surgery as part of a prospective, multi-center U.S. clinical trial.
Study findings demonstrate that, even at a premium of $4,000 over the cost of a TLIF implant, TOPS still achieves cost-effectiveness at a willingness-to-pay threshold of $100,000 within one year after surgery. Furthermore, TOPS becomes the dominant strategy when data is examined at two years and beyond. From the societal perspective, TOPS is even more highly cost-effective at one year and dominant at two years and beyond.
TOPS has been given Breakthrough Device Designation from FDA and is currently the subject of a pivotal clinical trial under an investigational device exemption from FDA.
“We developed TOPS to address a significant unmet need in the treatment of spinal stenosis and spondylolisthesis, and this study data demonstrate that TOPS not only provides clinical benefit to patients but also cuts sizeable treatment costs to hospital providers and payers,” said Premia Spine CEO Ron Sacher. “TOPS is well poised to be a win-win for patients, surgeons, facilities, and payers.”
Source: Premia Spine
Premia Spine announced clinical data demonstrating that the TOPS Spinal Arthroplasty System provides significant health system and societal benefit vs. transforaminal lumbar interbody fusion (TLIF). Reported to be the first and only facet joint replacement system for the lumbar spine, TOPS was developed to provide lasting mobility, stability and...
Premia Spine announced clinical data demonstrating that the TOPS Spinal Arthroplasty System provides significant health system and societal benefit vs. transforaminal lumbar interbody fusion (TLIF). Reported to be the first and only facet joint replacement system for the lumbar spine, TOPS was developed to provide lasting mobility, stability and durability after decompression for patients with lumbar spinal stenosis and degenerative spondylolisthesis.
The study explored the cost-effectiveness and economic analysis of TOPS versus TLIF for patients with spondylolisthesis and spinal stenosis. Patients were randomly assigned to either TOPS or TLIF surgery as part of a prospective, multi-center U.S. clinical trial.
Study findings demonstrate that, even at a premium of $4,000 over the cost of a TLIF implant, TOPS still achieves cost-effectiveness at a willingness-to-pay threshold of $100,000 within one year after surgery. Furthermore, TOPS becomes the dominant strategy when data is examined at two years and beyond. From the societal perspective, TOPS is even more highly cost-effective at one year and dominant at two years and beyond.
TOPS has been given Breakthrough Device Designation from FDA and is currently the subject of a pivotal clinical trial under an investigational device exemption from FDA.
“We developed TOPS to address a significant unmet need in the treatment of spinal stenosis and spondylolisthesis, and this study data demonstrate that TOPS not only provides clinical benefit to patients but also cuts sizeable treatment costs to hospital providers and payers,” said Premia Spine CEO Ron Sacher. “TOPS is well poised to be a win-win for patients, surgeons, facilities, and payers.”
Source: Premia Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.